Impaired dopamine metabolism in Parkinson's disease pathogenesis - PubMed (original) (raw)
Review
Impaired dopamine metabolism in Parkinson's disease pathogenesis
Anna Masato et al. Mol Neurodegener. 2019.
Abstract
A full understanding of Parkinson's Disease etiopathogenesis and of the causes of the preferential vulnerability of nigrostriatal dopaminergic neurons is still an unsolved puzzle. A multiple-hit hypothesis has been proposed, which may explain the convergence of familial, environmental and idiopathic forms of the disease. Among the various determinants of the degeneration of the neurons in Substantia Nigra pars compacta, in this review we will focus on the endotoxicity associated to dopamine dyshomeostasis. In particular, we will discuss the relevance of the reactive dopamine metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL) in the catechol-induced neurotoxicity. Indeed, the synergy between the catechol and the aldehyde moieties of DOPAL exacerbates its reactivity, resulting in modification of functional protein residues, protein aggregation, oxidative stress and cell death. Interestingly, αSynuclein, whose altered proteostasis is a recurrent element in Parkinson's Disease pathology, is considered a preferential target of DOPAL modification. DOPAL triggers αSynuclein oligomerization leading to synapse physiology impairment. Several factors can be responsible for DOPAL accumulation at the pre-synaptic terminals, i.e. dopamine leakage from synaptic vesicles, increased rate of dopamine conversion to DOPAL by upregulated monoamine oxidase and decreased DOPAL degradation by aldehyde dehydrogenases. Various studies report the decreased expression and activity of aldehyde dehydrogenases in parkinsonian brains, as well as genetic variants associated to increased risk in developing the pathology. Thus, we discuss how the deregulation of these enzymes might be considered a contributing element in the pathogenesis of Parkinson's Disease or a down-stream effect. Finally, we propose that a better understanding of the impaired dopamine metabolism in Parkinson's Disease would allow a more refined patients stratification and the design of more targeted and successful therapeutic strategies.
Keywords: Aldehyde dehydrogenase; DOPAL; Dopamine; Parkinson’s disease; Selective vulnerability; αSynuclein.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
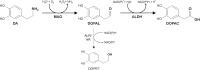
Fig. 1
Dopamine catabolism. In dopaminergic neurons, DA catabolism starts with deamination by MAO to generate DOPAL. The aldehyde moiety is then converted to the carboxyl group of DOPAC by ALDHs. A smaller fraction of DOPAL aldehyde is converted to the hydroxyl group of DOPET by ALR/ARs (thinner arrow)
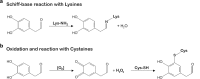
Fig. 2
DOPAL reactivity and reported neurotoxic molecular mechanisms. DOPAL reactivity is due to both the aldehyde and the catechol moiety, respectively resulting in covalent modification of primary amines and thiols (i.e. lysine and cysteine residues of proteins) [–38]. a DOPAL addiction to lysines is the result of a Schiff-base reaction between the aldehyde and the primary amine of the lysine’s lateral chain, with the release of a molecule of water. b In oxidative conditions, the catechol group has the tendency to auto-oxidation, with production of quinones and oxygen radical species [39]. Also, the oxidized cathecol is reactive towards the thiols of cysteines
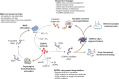
Fig. 3
DOPAL reported neurotoxic molecular mechanisms. DOPAL build-up in SNpc dopaminergic neurons triggers multiple neurotoxic mechanisms: a alteration of neuronal proteostasis, in terms of protein aggregation [34, 36, 38, 41, 44], competition with functional post-translational modifications (PTMs, i.e. ubiquitination, SUMOylation, acetylation) and accumulation of ubiquitinated proteins [42, 45]; b enzyme inhibition (PDB: 4i1f, in the figure) [–48]; c indirect effects, which imply oxidative stress [39], mitochondrial dysfunction [, –51], activation of necrotic and apoptotic pathways [23, 24, 33]
Fig. 4
Potential interplay between DOPAL and αSynuclein at pre-synaptic terminals and determinants of DOPAL accumulation. DOPAL accumulation at the pre-synaptic terminals covalently modifies αSyn lysines, reducing αSyn affinity for membrane binding and resulting in synaptic vesicles pools redistribution [38, 41]. αSyn-DOPAL oligomers accumulate and permeabilize synaptic vesicles membrane [41], leading to cytosolic DA release, which is further metabolized into DOPAL by MAO. Also, DOPAL activates AEP (PDB: 4aw9, in the figure), which cleaves αSyn at N103 [76]. Truncated αSyn is more prone to aggregation and stimulates MAO activity. Hence, the result is a positive loop that self amplifies, leading to αSyn aggregation and synapse degeneration. In the figure, the black thin arrows indicate the chemical reactions, while the thicker ones highlight the cellular processes. Among the factors that could lead to DOPAL build-up, the critical hubs are the dysfunction of DA storage in synaptic vesicles, increased rate of DA degradation by MAO and decreased DOPAL detoxification by ALDHs. For each point, the evidences are listed in the figure
Fig. 5
Effects of αSynuclein dyshomeostasis on synapse functionality. Under physiological conditions, αSyn ensures the correct balance of DA release in the striatum by binding to synaptic vesicles membrane, regulating vesicles mobility and the exocytotic events. However, upon αSyn dyshomeostasis, which includes both αSyn accumulation or its absence, the synaptic vesicles distribution among the different pools and the neurotransmitter release are altered, as demonstrated in the Syn-TKO and the αSyn-OVX mouse models [, , –84]. Conversely, the DOPAL-αSyn interplay presents an additional level of complexity. Indeed, DOPAL modification of αSyn lysines hinders its association to synaptic vesicles membrane, mimicking a KO-like phenotype [38]. At the same time, DOPAL triggers αSyn aggregation in off-pathway pore-forming oligomers, resulting in synaptic vesicles permeabilization [41]. Furthermore, DOPAL build-up induces synaptic vesicles clustering of the resting pool, resembling the αSyn-overexpressing scenario [41]
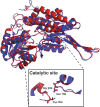
Fig. 6
ALDH1A1 and ALDH2 structures. Superimposition of ALDH1A1 (PDB: 5L2O, in blue) and ALDH2 (PDB: 1O02, in red) subunit structures. In the box, the spatial orientation of the conserved residues in the catalytic site (Asn169, Gly299, Cys302) is reported
Similar articles
- Oligomerization and Membrane-binding Properties of Covalent Adducts Formed by the Interaction of α-Synuclein with the Toxic Dopamine Metabolite 3,4-Dihydroxyphenylacetaldehyde (DOPAL).
Follmer C, Coelho-Cerqueira E, Yatabe-Franco DY, Araujo GD, Pinheiro AS, Domont GB, Eliezer D. Follmer C, et al. J Biol Chem. 2015 Nov 13;290(46):27660-79. doi: 10.1074/jbc.M115.686584. Epub 2015 Sep 17. J Biol Chem. 2015. PMID: 26381411 Free PMC article. - Rotenone decreases intracellular aldehyde dehydrogenase activity: implications for the pathogenesis of Parkinson's disease.
Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Kopin IJ, Sharabi Y. Goldstein DS, et al. J Neurochem. 2015 Apr;133(1):14-25. doi: 10.1111/jnc.13042. Epub 2015 Feb 25. J Neurochem. 2015. PMID: 25645689 Free PMC article. - DOPAL initiates αSynuclein-dependent impaired proteostasis and degeneration of neuronal projections in Parkinson's disease.
Masato A, Plotegher N, Terrin F, Sandre M, Faustini G, Thor A, Adams S, Berti G, Cogo S, De Lazzari F, Fontana CM, Martinez PA, Strong R, Bandopadhyay R, Bisaglia M, Bellucci A, Greggio E, Dalla Valle L, Boassa D, Bubacco L. Masato A, et al. NPJ Parkinsons Dis. 2023 Mar 25;9(1):42. doi: 10.1038/s41531-023-00485-1. NPJ Parkinsons Dis. 2023. PMID: 36966140 Free PMC article. - Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons.
Doorn JA, Florang VR, Schamp JH, Vanle BC. Doorn JA, et al. Parkinsonism Relat Disord. 2014 Jan;20 Suppl 1(0 1):S73-5. doi: 10.1016/S1353-8020(13)70019-1. Parkinsonism Relat Disord. 2014. PMID: 24262193 Free PMC article. Review. - The Catecholaldehyde Hypothesis for the Pathogenesis of Catecholaminergic Neurodegeneration: What We Know and What We Do Not Know.
Goldstein DS. Goldstein DS. Int J Mol Sci. 2021 Jun 1;22(11):5999. doi: 10.3390/ijms22115999. Int J Mol Sci. 2021. PMID: 34206133 Free PMC article. Review.
Cited by
- Small Molecules, α-Synuclein Pathology, and the Search for Effective Treatments in Parkinson's Disease.
Sechi GP, Sechi MM. Sechi GP, et al. Int J Mol Sci. 2024 Oct 18;25(20):11198. doi: 10.3390/ijms252011198. Int J Mol Sci. 2024. PMID: 39456980 Free PMC article. Review. - Role of Peroxynitrite in the Pathogenesis of Parkinson's Disease and Its Fluorescence Imaging-Based Detection.
Lv J, Chen F, Zhang C, Kang Y, Yang Y, Zhang C. Lv J, et al. Biosensors (Basel). 2024 Oct 17;14(10):506. doi: 10.3390/bios14100506. Biosensors (Basel). 2024. PMID: 39451719 Free PMC article. Review. - Visual Detection of Dopamine with CdS/ZnS Quantum Dots Bearing by ZIF-8 and Nanofiber Membranes.
Hu J, Li J, Guo Q, Du G, Li C, Li R, Zhou R, He H. Hu J, et al. Int J Mol Sci. 2024 Sep 26;25(19):10346. doi: 10.3390/ijms251910346. Int J Mol Sci. 2024. PMID: 39408675 Free PMC article. - Characterizing the adult zebrafish model of Parkinson's disease: a systematic review of dynamic changes in behavior and physiology post-MPTP administration.
Razali K, Kumar J, Mohamed WMY. Razali K, et al. Front Neurosci. 2024 Sep 10;18:1432102. doi: 10.3389/fnins.2024.1432102. eCollection 2024. Front Neurosci. 2024. PMID: 39319314 Free PMC article. - Neural stem cells derived from α-synuclein-knockdown iPS cells alleviate Parkinson's disease.
Wang CH, Lin GC, Fu RH, Huang YC, Chen SY, Lin SZ, Harn HJ, Shyu WC, Huang YF, Jeng LB, Liu SP. Wang CH, et al. Cell Death Discov. 2024 Sep 17;10(1):407. doi: 10.1038/s41420-024-02176-z. Cell Death Discov. 2024. PMID: 39285205 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous