Growling from the gut: co-option of the gastric mill for acoustic communication in ghost crabs - PubMed (original) (raw)
Growling from the gut: co-option of the gastric mill for acoustic communication in ghost crabs
Jennifer R A Taylor et al. Proc Biol Sci. 2019.
Abstract
Animal acoustic communication systems can be built upon co-opted structures that become specialized for sound production or morphological novelties. The ghost crab, Ocypode quadrata, evolved a novel stridulation apparatus on the claws that is used during agonistic interactions, but they also produce a rasping sound without their claw apparatus. We investigated the nature of these sounds and show that O. quadrata adopted a unique and redundant mode of sound production by co-opting the gastric mill (grinding teeth of the foregut). Acoustic characteristics of the sound are consistent with stridulation and are produced by both male and female crabs during aggressive interactions. Laser Doppler vibrometry localized the source of maximum vibration to the gastric region and fluoroscopy showed movement of the gastric mill that coincided with stridulation. The lateral teeth of the gastric mill possess a series of comb-like structures that rub against the median tooth to produce stridulation with dominant frequencies below 2 kHz. This previously undescribed gastric stridulation can be modulated and provide a means of assessment during aggressive interactions, similar to the use of the claw stridulation apparatus. This functional redundancy of stridulation in crabs offers unique insights into the mechanisms of evolution of acoustic communication systems.
Keywords: Crustacea; Ocypode; animal behaviour; bioacoustics; functional morphology.
Conflict of interest statement
The authors declare no competing interests.
Figures
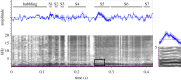
Figure 1.
Acoustic recording of a ghost crab bubbling and stridulating. Oscillogram (top) shows consistently spaced pulses within each rasp of stridulation but not during bubbling. Spectrogram (bottom) shows seven stridulations, or rasps, with most of the energy below 2 kHz and visible harmonics at high pulse rates. Rasp duration, pulse number, and pulse rates are highly variable within an individual (S1–S7). The boxed region of a rasp is magnified on the right to show harmonics with greatest energy.
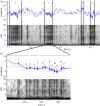
Figure 2.
Characteristics of gastric mill stridulation. A bout of stridulation (a) composed of four rasps, each of different duration (noted by width of the column). Rasp frequency was calculated as the number of rasps divided by the duration of stridulation bout (11.4 Hz in this example). Magnification of an individual rasp (b) revealing individual pulses. Each pulse represents a ridge of the comb-like structures on the lateral tooth rubbing against the medial tooth. Pulse rate was calculated as the number of pulses divided by the duration of the rasp (481 Hz in this example).
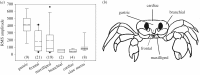
Figure 3.
(a,b) LDV signals from ghost crabs. (a) Amplitude of the vibration signal was significantly greater in the gastric region compared to all other regions. Number of signals analysed, N, noted in parentheses. Solid line, mean; dashed line, median. (b) Regions of crab measured with LDV.
Figure 4.
Ghost crab gastric (a_–_c) and claw (d) stridulation apparatus. (a) Microscope image showing lateral teeth in open position, apart from the medial tooth. (b) Lateral tooth with 17 small comb-like teeth and arrow showing direction of movement along the medial tooth. (c) CT scan of intact gastric mill showing mineralized ossicles and closed position of the lateral teeth against the medial tooth (antero-ventral view). L, lateral tooth; M, medial tooth. Scale bar, 2.5 mm. (d) Major claw showing the location of novel stridulation structures. To stridulate, crabs flex the cheliped to rub the pars stridens of the propodus across the plectrum on the ischium. Enlarged images show the ridge-like plectrum (p) and the 14 tubercles of the pars stridens (ps). I, ishium; M, merus; C, carpus; P, propodus.
Similar articles
- Morphology of the gastric mill teeth in dotillid crabs (Crustacea: Brachyura: Dotillidae) from Indonesia.
Murniati DC, Asakura A, Nakano T, Shimomura M. Murniati DC, et al. J Morphol. 2023 Jul;284(7):e21605. doi: 10.1002/jmor.21605. J Morphol. 2023. PMID: 37313771 - Acoustic defence in an insect: characteristics of defensive stridulation and differences between the sexes in the tettigoniid Poecilimon ornatus (Schmidt 1850).
Kowalski KN, Lakes-Harlan R, Lehmann GU, Strauß J. Kowalski KN, et al. Zoology (Jena). 2014 Oct;117(5):329-36. doi: 10.1016/j.zool.2014.04.007. Epub 2014 Aug 2. Zoology (Jena). 2014. PMID: 25156932 - Mechanisms of high-frequency song generation in brachypterous crickets and the role of ghost frequencies.
Robillard T, Montealegre-Z F, Desutter-Grandcolas L, Grandcolas P, Robert D. Robillard T, et al. J Exp Biol. 2013 Jun 1;216(Pt 11):2001-11. doi: 10.1242/jeb.083964. Epub 2013 Feb 21. J Exp Biol. 2013. PMID: 23430987 - Sexual selection and predation drive the repeated evolution of stridulation in Heteroptera and other arthropods.
Davranoglou LR, Taylor GK, Mortimer B. Davranoglou LR, et al. Biol Rev Camb Philos Soc. 2023 Jun;98(3):942-981. doi: 10.1111/brv.12938. Epub 2023 Feb 14. Biol Rev Camb Philos Soc. 2023. PMID: 36787892 Review. - Acoustic detection and communication by decapod crustaceans.
Popper AN, Salmon M, Horch KW. Popper AN, et al. J Comp Physiol A. 2001 Mar;187(2):83-9. doi: 10.1007/s003590100184. J Comp Physiol A. 2001. PMID: 15523997 Review.
Cited by
- Acoustic particle motion detection in the snapping shrimp (Alpheus richardsoni).
Dinh JP, Radford C. Dinh JP, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2021 Sep;207(5):641-655. doi: 10.1007/s00359-021-01503-4. Epub 2021 Jul 9. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2021. PMID: 34241712 - Evolutionary novelty in communication between the sexes.
Broder ED, Elias DO, Rodríguez RL, Rosenthal GG, Seymoure BM, Tinghitella RM. Broder ED, et al. Biol Lett. 2021 Feb;17(2):20200733. doi: 10.1098/rsbl.2020.0733. Epub 2021 Feb 3. Biol Lett. 2021. PMID: 33529546 Free PMC article.
References
- Gould SJ, Vrba ES. 1982. Exaptation—a missing term in the science of form. Paleobiology 8, 4–15. (10.1017/S0094837300004310) - DOI
- McLennan DA. 2008. The concept of co-option: why evolution often looks miraculous. Evol. Educ. Outreach 1, 247–258. (10.1007/s12052-008-0053-8) - DOI
- Parmentier E, Diogo R, Fine ML. 2017. Multiple exaptations leading to fish sound production. Fish Fish. 18, 958–966. (10.1111/faf.12217) - DOI
- Dumortier B. 1963. Morphology of sound emission apparatus in Arthropoda. In Acoustic behaviour of animals (ed. Busnel R-G.), pp. 277–345. Amsterdam, The Netherlands: Elsevier.