APOL1 Kidney Risk Variants Induce Cell Death via Mitochondrial Translocation and Opening of the Mitochondrial Permeability Transition Pore - PubMed (original) (raw)
APOL1 Kidney Risk Variants Induce Cell Death via Mitochondrial Translocation and Opening of the Mitochondrial Permeability Transition Pore
Shrijal S Shah et al. J Am Soc Nephrol. 2019 Dec.
Abstract
Background: Genetic Variants in Apolipoprotein L1 (APOL1) are associated with large increases in CKD rates among African Americans. Experiments in cell and mouse models suggest that these risk-related polymorphisms are toxic gain-of-function variants that cause kidney dysfunction, following a recessive mode of inheritance. Recent data in trypanosomes and in human cells indicate that such variants may cause toxicity through their effects on mitochondria.
Methods: To examine the molecular mechanisms underlying APOL1 risk variant-induced mitochondrial dysfunction, we generated tetracycline-inducible HEK293 T-REx cells stably expressing the APOL1 nonrisk G0 variant or APOL1 risk variants. Using these cells, we mapped the molecular pathway from mitochondrial import of APOL1 protein to APOL1-induced cell death with small interfering RNA knockdowns, pharmacologic inhibitors, blue native PAGE, mass spectrometry, and assessment of mitochondrial permeability transition pore function.
Results: We found that the APOL1 G0 and risk variant proteins shared the same import pathway into the mitochondrial matrix. Once inside, G0 remained monomeric, whereas risk variant proteins were prone to forming higher-order oligomers. Both nonrisk G0 and risk variant proteins bound components of the mitochondrial permeability transition pore, but only risk variant proteins activated pore opening. Blocking mitochondrial import of APOL1 risk variants largely eliminated oligomer formation and also rescued toxicity.
Conclusions: Our study illuminates important differences in the molecular behavior of APOL1 nonrisk and risk variants, and our observations suggest a mechanism that may explain the very different functional effects of these variants, despite the lack of consistently observed differences in trafficking patterns, intracellular localization, or binding partners. Variant-dependent differences in oligomerization pattern may underlie APOL1's recessive, gain-of-function biology.
Keywords: APOL1; chronic kidney disease; focal segmental glomerulosclerosis; genetic renal disease; mitochondria.
Copyright © 2019 by the American Society of Nephrology.
Figures
Graphical abstract
Figure 1.
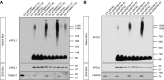
APOL1 translocates into the mitochondrial matrix. (A) APOL1 cofractionates with the mitochondrial marker TOMM20, indicating mitochondrial association. HEK293 cells stably transfected with APOL1 (EV, G0, G1, or G2) were induced with tetracycline for 6 hours. The cell lysate was separated by differential centrifugation into a mitochondria-enriched fraction (TOMM20 positive) and a supernatant fraction (tubulin positive). (B) APOL1 is translocated into mitochondria. Isolated mitochondria were subjected to Proteinase K digestion to degrade proteins bound to the OMM and MAM-associated proteins. Proteinase K digests the OMM protein TOMM20 and the MAM protein ACSL4 but not the IMM protein TIM23 or matrix protein HSP60. Continued presence of APOL1 after protease digestion indicates prior mitochondrial internalization. (C) APOL1 translocates to mitochondrial matrix. Isolated mitochondria were first incubated in hypotonic solution to lyse the OMM, then subjected to Proteinase K digestion. OMM (TOMM20), IMM (TIM23), and MAM (ACSL4) proteins were digested, but matrix proteins HSP60 and APOL1 are protected. Addition of TritonX-100 digestion solubilized lipids of the IMM and rendered APOL1 in the mitochondrial matrix susceptible to digestion by Proteinase K.
Figure 2.
APOL1 translocation to mitochondrial matrix is dependent on IMM and OMM translocase machinery. (A and B) Knockdown of TOMM20 reduces mitochondrial APOL1. Nontarget siRNA (siNT) does not affect APOL1 translocation, as demonstrated by persistence of intact APOL1 in the presence of Proteinase K. Knockdown of TOMM20 (siTOMM20) substantially decreased mitochondrial APOL1 content after Proteinase K treatment. (C) Knockdown of TOMM20 and TOMM22 in matrix protein translocation pathway reduces RV APOL1-induced cell death. In contrast, knockdown of TOMM70, an OMM protein involved in targeting proteins to the IMM in the carrier pathway, does not affect RV APOL1-induced cell death. TOMM70 knockdown efficiency is shown in
Supplemental Figure 6B
. (D) Knockdown of TIMM17 and TIMM23 in the matrix protein translocation pathway and knockdown of mitochondrial matrix translocation chaperone HSPA9 independently reduced APOL1-induced cell death. In contrast, knockdown of the carrier pathway protein TIMM22 that shuttles incoming proteins into the IMM does not affect APOL1-induced cell death. TIMM22 knockdown efficiency is shown in
Supplemental Figure 6C
. (A–D) Stably transfected HEK293 cells were treated with 50 ng/ml of tetracycline for 6 (A and B) or 18 (C and D) hours. (B) Densitometry was performed on three independent experiments. (C and D) Each dot represents an independent experiment. **P<0.001 comparing each siRNA to siNT-treated cells.
Figure 3.
APOL1 RVs form higher-ordered oligomers that are enriched in the mitochondria. (A) Soluble fractions from digitonin lysates of APOL1-expressing cells were subjected to native PAGE, demonstrating that RV APOL1 forms a range of higher-ordered oligomers. (B) Differential centrifugation with mitochondrial isolation followed by digitonin lysis and native PAGE demonstrate that higher-order RV APOL1 is present primarily in the mitochondrial enriched fraction. Discrete oligomers appear at up to 1.2 Md; larger aggregates are retained in the well at top. (A and B) Stably transfected HEK293 cells expressing APOL1 were induced for 15 hours with (A) 25 ng/ml tetracycline or (B) 6 hours with 50 ng/ml tetracycline before lysis.
Figure 4.
APOL1 transport to mitochondrial matrix is required for formation of higher-order oligomers. (A and B) Knockdown of TIMM17 (a key component of the IMM protein translocation machinery) or HSPA9 (a mitochondrial matrix translocation chaperone needed to guide proteins through the translocation machinery) prevents APOL1 higher-order oligomer formation (in addition to preventing cell death: see Figure 2D). Conversely, knockdown of TIMM22 affects neither cell death nor APOL1 oligomer formation (see
Supplemental Figure 6C
). Stable APOL1-transfected cells were treated for 48 hours with siRNA prior to inducing APOL1 with 50 ng/ml tetracycline. Cells were lysed with digitonin after 15 hours and were fractionated by native PAGE (large top panels) or SDS page (lower narrow panels). The signal from the monomeric fraction is saturated in these images.
Figure 5.
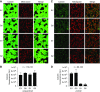
APOL1 RVs induce MPTP opening (A). Calcein staining before CoCl2 quench was not different between EV, G0, G1 and G2 cells. (B) Quantification of calcein fluorescence before addition of cobalt chloride. Data expressed as mean±SD, for “_n_” cells subjected to quantification. (C) Calcein-AM staining with CoCl2 quenching indicates that APOL1 RVs cause opening of the mPTP. Calcein-AM dye diffuses into and throughout the cell and is activated upon esterase cleavage of the acetoxymethyl ester group in live cells (green staining). CoCl2 enters the cell but, under normal conditions, cannot enter the mitochondria. mPTP activation leads to pore formation and allows cobalt chloride to enter the mitochondria. Confocal imaging demonstrates decreased calcein fluorescence in G1- and G2-expressing cells after CoCl2 quenching, indicating MPTP pore opening. Images shown are maximum intensity projections of a representative _z_-stack. (D) Quantification of mPTP pore induction as a function of APOL1 genotype, as measured by calcein fluorescence after CoCl2 as described in (B) above. Data expressed as mean±SD, for “_n_” cells subjected to quantification. Original magnification, X630.
Figure 6.
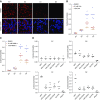
APOL1 RVs depolarize mitochondrial potential and induce cytotoxicity, reversible with pharmacologic inhibition or knockdown of mPTP components. (A) TMRM dye accumulates in the mitochondrial matrix on the basis of the hyperpolarized mitochondrial transmembrane potential. Compared with EV and G0 cells, TMRM fluorescence is much lower in APOL1 RV-expressing cells, indicating reduced mitochondrial transmembrane potential in RV cells. Original magnification, X630. (B) CYPD inhibitor CsA blocks mPTP formation and reduces APOL1 RV–induced cell death. Each dot represents an independent experiment. For both G1 and G2 cell lines, DMSO versus 0.1 _µ_M CsA and DMSO versus 1 _µ_M CsA; P<0.001. (C) NIM811, a CsA analog that can inhibit MPTP by binding to CYPD but cannot bind to calcineurin, blocks APOL1 RV induced cell death. Each dot represents an independent experiment. For both G1 and G2 cell lines, DMSO versus 0.1 _µ_M NIM811 and DMSO versus 1 _µ_M NIM811; P<0.001. (D) Knockdown of individual MPTP pore components or modulators blocks APOL1-induced cell death.
Supplemental Figure 8
includes immunoprecipitation Western blots demonstrating interaction between APOL1 and MPTP components. Each dot represents an independent experiment. **P<0.001 comparing each siRNA to siNT-treated cells.
Figure 7.
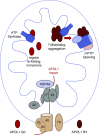
Model for differential toxicity of G0 and RV APOL1. APOL1 G0 and RVs enter the mitochondrial matrix via the same import machinery. Although they remain mostly monomeric, RVs tend to form higher-order oligomers after import. Whereas all APOL1 variants appear to bind components or regulators of the mPTP, the binding of aggregated RVs (likely in oligomeric form) activates pore opening, mitochondrial dysfunction, and cell death. Because proteins generally unfold before mitochondrial import, one possibility is that G0 refolds normally after import and remains monomeric whereas RVs refold abnormally and have a propensity to aggregate.
Comment in
- APOL1 risk variants induce opening of the mitochondrial permeability transition pore.
Carney EF. Carney EF. Nat Rev Nephrol. 2019 Dec;15(12):730. doi: 10.1038/s41581-019-0222-8. Nat Rev Nephrol. 2019. PMID: 31602044 No abstract available.
Similar articles
- Kidney Disease-Associated APOL1 Variants Have Dose-Dependent, Dominant Toxic Gain-of-Function.
Datta S, Kataria R, Zhang JY, Moore S, Petitpas K, Mohamed A, Zahler N, Pollak MR, Olabisi OA. Datta S, et al. J Am Soc Nephrol. 2020 Sep;31(9):2083-2096. doi: 10.1681/ASN.2020010079. Epub 2020 Jul 16. J Am Soc Nephrol. 2020. PMID: 32675303 Free PMC article. - An Acidic Environment Induces APOL1-Associated Mitochondrial Fragmentation.
Li D, Snipes JA, Murea M, Molina AJA, Divers J, Freedman BI, Ma L, Petrovic S. Li D, et al. Am J Nephrol. 2020;51(9):695-704. doi: 10.1159/000509989. Epub 2020 Aug 31. Am J Nephrol. 2020. PMID: 32866949 Free PMC article. - Recessive, gain-of-function toxicity in an APOL1 BAC transgenic mouse model mirrors human APOL1 kidney disease.
McCarthy GM, Blasio A, Donovan OG, Schaller LB, Bock-Hughes A, Magraner JM, Suh JH, Tattersfield CF, Stillman IE, Shah SS, Zsengeller ZK, Subramanian B, Friedman DJ, Pollak MR. McCarthy GM, et al. Dis Model Mech. 2021 Aug 1;14(8):dmm048952. doi: 10.1242/dmm.048952. Epub 2021 Aug 5. Dis Model Mech. 2021. PMID: 34350953 Free PMC article. - APOL1 and kidney cell function.
Kumar V, Singhal PC. Kumar V, et al. Am J Physiol Renal Physiol. 2019 Aug 1;317(2):F463-F477. doi: 10.1152/ajprenal.00233.2019. Epub 2019 Jun 26. Am J Physiol Renal Physiol. 2019. PMID: 31241995 Free PMC article. Review. - Ten years in: APOL1 reaches beyond the kidney.
Waitzman JS, Lin J. Waitzman JS, et al. Curr Opin Nephrol Hypertens. 2019 Jul;28(4):375-382. doi: 10.1097/MNH.0000000000000511. Curr Opin Nephrol Hypertens. 2019. PMID: 31082862 Free PMC article. Review.
Cited by
- Collapsing Glomerulopathy.
Koirala A, Akilesh S, Jefferson JA. Koirala A, et al. Adv Kidney Dis Health. 2024 Jul;31(4):290-298. doi: 10.1053/j.akdh.2024.03.008. Adv Kidney Dis Health. 2024. PMID: 39084754 Review. - Kidney Disease-Associated APOL1 Variants Have Dose-Dependent, Dominant Toxic Gain-of-Function.
Datta S, Kataria R, Zhang JY, Moore S, Petitpas K, Mohamed A, Zahler N, Pollak MR, Olabisi OA. Datta S, et al. J Am Soc Nephrol. 2020 Sep;31(9):2083-2096. doi: 10.1681/ASN.2020010079. Epub 2020 Jul 16. J Am Soc Nephrol. 2020. PMID: 32675303 Free PMC article. - An Acidic Environment Induces APOL1-Associated Mitochondrial Fragmentation.
Li D, Snipes JA, Murea M, Molina AJA, Divers J, Freedman BI, Ma L, Petrovic S. Li D, et al. Am J Nephrol. 2020;51(9):695-704. doi: 10.1159/000509989. Epub 2020 Aug 31. Am J Nephrol. 2020. PMID: 32866949 Free PMC article. - APOL1 Risk Variants Impair Multiple Mitochondrial Pathways in a Metabolomics Analysis.
Ma L, Palmer ND, Choi YA, Murea M, Snipes JA, Parks JS, Langefeld CD, Freedman BI. Ma L, et al. Kidney360. 2020 Sep 30;1(12):1353-1362. doi: 10.34067/KID.0003592020. eCollection 2020 Dec 31. Kidney360. 2020. PMID: 35372896 Free PMC article. - The glomerular filtration barrier: a structural target for novel kidney therapies.
Daehn IS, Duffield JS. Daehn IS, et al. Nat Rev Drug Discov. 2021 Oct;20(10):770-788. doi: 10.1038/s41573-021-00242-0. Epub 2021 Jul 14. Nat Rev Drug Discov. 2021. PMID: 34262140 Free PMC article. Review.
References
- Duchateau PN, Pullinger CR, Cho MH, Eng C, Kane JP: Apolipoprotein L gene family: Tissue-specific expression, splicing, promoter regions; discovery of a new gene. J Lipid Res 42: 620–630, 2001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous