Cyclic Polyhydroxy Ketones. I. Oxidation Products of Hexahydroxybenzene (Benzenehexol) - PubMed (original) (raw)
Cyclic Polyhydroxy Ketones. I. Oxidation Products of Hexahydroxybenzene (Benzenehexol)
Alexander J Fatiadi et al. J Res Natl Bur Stand A Phys Chem. 1963 Mar-Apr.
Abstract
Reliable procedures are given for the preparation and purification of hexahydroxybenzene (benzenehexol), tetrahydroxy-_p_-benzoquinone, rhodizonic acid, triquinoyl (cyclohexanehexone), croconic acid, and leuconic acid (cyclopentanepentone). Certain derivatives and color tests, as well as infrared and ultraviolet spectra, are reported for their identification.
Figures
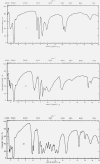
Figure 1.. Infrared spectrograms of materials in Nujol mulls.
I, Hexahydroxybenzene; II, tetrahydroxyquinone; III, rhodizonic acid dihydrate. IV, Triquinoyl octahydrate; V, croconic acid trihydratc; VI, leuconic acid pentahydrate.
Figure 1.. Infrared spectrograms of materials in Nujol mulls.
I, Hexahydroxybenzene; II, tetrahydroxyquinone; III, rhodizonic acid dihydrate. IV, Triquinoyl octahydrate; V, croconic acid trihydratc; VI, leuconic acid pentahydrate.
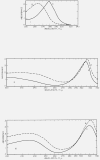
Figure 2.. Ultraviolet spectrograms of materials.
I. Hexahydroxybenzene. Curve A, 7.39 mg of I in 100 ml of 6_N_ HCl in methanol, 3 min after dissolution; λmax at 278 m_μ_. Curve B, 67 mg of the hexaacetate of I in 200 ml of glacial acetic acid, 10 min after dissolution; λmax at 263 m_μ_. II. Tetrahydroxyquinone. Curve A, 3.82 mg of II in 1000 ml of 2_N_ aqueous HCl, 24 hr after dissolution; λmax at 308 m_μ_. Curve B, 7 mg of II in 1000 ml of methanol, 30 min after dissolution; λmax at 312 m_μ_. III. Rhodizonic acid dihydrate. Curve A, 6.87 mg of III in 1000 ml of 2_N_ aqueous HCl 24 hr after dissolution; λmax at 235 m_μ_ and at 319 m_μ_. Curve B, 8.58 mg of III in 1000 ml of methanol, 5 min after dissolution; λmax at 235 m_μ_ and at 323 m_μ_. In the visible region, III shows absorption at 443 m_μ_ and at 487 m_μ_ (sh). IV. Triquinoyl octahydrate. 3.24 mg of IV in 100 ml of 2_N_ aqueous NaCl 1.5 hr after dissolution; λmax at 267 mμ. In the visible region, IV shows marked absorption at 363 m_μ_, 440 m_μ_ (sh) and 480 m_μ_. V. Croconic acid trihydrate. 5.87 mg of V in 100 ml of 2_N_ aqueous HCl, 10 min after dissolution; λmax at 231 m_μ_ and at 298 m_μ_. VI. Leuconic acid pentahydrate. 156.45 mg of VI in 50 ml of water, 5 min after dissolution; λmax at 328 m_μ_.
Figure 2.. Ultraviolet spectrograms of materials.
I. Hexahydroxybenzene. Curve A, 7.39 mg of I in 100 ml of 6_N_ HCl in methanol, 3 min after dissolution; λmax at 278 m_μ_. Curve B, 67 mg of the hexaacetate of I in 200 ml of glacial acetic acid, 10 min after dissolution; λmax at 263 m_μ_. II. Tetrahydroxyquinone. Curve A, 3.82 mg of II in 1000 ml of 2_N_ aqueous HCl, 24 hr after dissolution; λmax at 308 m_μ_. Curve B, 7 mg of II in 1000 ml of methanol, 30 min after dissolution; λmax at 312 m_μ_. III. Rhodizonic acid dihydrate. Curve A, 6.87 mg of III in 1000 ml of 2_N_ aqueous HCl 24 hr after dissolution; λmax at 235 m_μ_ and at 319 m_μ_. Curve B, 8.58 mg of III in 1000 ml of methanol, 5 min after dissolution; λmax at 235 m_μ_ and at 323 m_μ_. In the visible region, III shows absorption at 443 m_μ_ and at 487 m_μ_ (sh). IV. Triquinoyl octahydrate. 3.24 mg of IV in 100 ml of 2_N_ aqueous NaCl 1.5 hr after dissolution; λmax at 267 mμ. In the visible region, IV shows marked absorption at 363 m_μ_, 440 m_μ_ (sh) and 480 m_μ_. V. Croconic acid trihydrate. 5.87 mg of V in 100 ml of 2_N_ aqueous HCl, 10 min after dissolution; λmax at 231 m_μ_ and at 298 m_μ_. VI. Leuconic acid pentahydrate. 156.45 mg of VI in 50 ml of water, 5 min after dissolution; λmax at 328 m_μ_.
Similar articles
- Cyclic Polyhydroxy Ketones II. _xylo_-Trihydroxycyclohexenediolic Acid and Keto-Inositols.
Fatiadi AJ, Isbell HS. Fatiadi AJ, et al. J Res Natl Bur Stand A Phys Chem. 1964 May-Jun;68A(3):287-299. doi: 10.6028/jres.068A.026. Epub 1964 Jun 1. J Res Natl Bur Stand A Phys Chem. 1964. PMID: 31834723 Free PMC article. - Oxidation-reduction potentials and ionization constants of the reversible series; hexahydroxybenzene-tetrahydroxyquinone-rhodizonic acid.
PREISLER PW, BERGER L, HILL ES. PREISLER PW, et al. J Am Chem Soc. 1948 Feb;70(2):871. doi: 10.1021/ja01182a515. J Am Chem Soc. 1948. PMID: 18933297 No abstract available. - Prooxidant action of rhodizonic acid: transition metal-dependent generation of reactive oxygen species causing the formation of 8-hydroxy-2'-deoxyguanosine formation in DNA.
Murakami K, Haneda M, Naruse M, Yoshino M. Murakami K, et al. Toxicol In Vitro. 2006 Sep;20(6):910-4. doi: 10.1016/j.tiv.2006.01.009. Epub 2006 Feb 28. Toxicol In Vitro. 2006. PMID: 16504460 - New developments in oxidative fermentation.
Adachi O, Moonmangmee D, Toyama H, Yamada M, Shinagawa E, Matsushita K. Adachi O, et al. Appl Microbiol Biotechnol. 2003 Feb;60(6):643-53. doi: 10.1007/s00253-002-1155-9. Epub 2002 Dec 18. Appl Microbiol Biotechnol. 2003. PMID: 12664142 Review. - Recent methods for the synthesis of α-acyloxy ketones.
Prasad PK, Reddi RN, Arumugam S. Prasad PK, et al. Org Biomol Chem. 2018 Dec 12;16(48):9334-9348. doi: 10.1039/c8ob02881h. Org Biomol Chem. 2018. PMID: 30516787 Review.
Cited by
- Pseudo-Oxocarbons. Synthesis of 2, 1,3-Bis-, and 1, 2, 3-Tris (Dicyanomethylene) Croconate Salts. New Bond-Delocalized Dianions, "Croconate Violet" and "Croconate Blue".
Fatiadi AJ. Fatiadi AJ. J Res Natl Bur Stand (1977). 1980 Mar-Apr;85(2):73-86. doi: 10.6028/jres.085.005. J Res Natl Bur Stand (1977). 1980. PMID: 34566013 Free PMC article. - Carbon Monoxide Coupling Reactions: A New Concept for the Formation of Hexahydroxybenzene.
Rosenthal U. Rosenthal U. Chemistry. 2020 Nov 17;26(64):14507-14511. doi: 10.1002/chem.202001947. Epub 2020 Sep 18. Chemistry. 2020. PMID: 32428364 Free PMC article. - Cyclic Polyhydroxy Ketones II. _xylo_-Trihydroxycyclohexenediolic Acid and Keto-Inositols.
Fatiadi AJ, Isbell HS. Fatiadi AJ, et al. J Res Natl Bur Stand A Phys Chem. 1964 May-Jun;68A(3):287-299. doi: 10.6028/jres.068A.026. Epub 1964 Jun 1. J Res Natl Bur Stand A Phys Chem. 1964. PMID: 31834723 Free PMC article.
References
- Posternak T., Helv. Chim. Acta 19, 1333 (1936); 24, 1045 (1941).
- Posternak T. and Deshusses J., Helv. Chim. Acta 44, 2089 (1961).
- Stanacev N. Z. and Kates M., J. Org. Chem. 26, 912 (1961).
- Isbell H. S., Ann. Rev. Biochem. 12, 213 (1943).
- Isbell H. S., J. Research NBS 32, 45 (1944).
LinkOut - more resources
Full Text Sources
Other Literature Sources