A Randomized Double-blind Placebo-controlled Trial on the Effect of Magnesium Oxide in Patients With Chronic Constipation - PubMed (original) (raw)
. 2019 Oct 30;25(4):563-575.
doi: 10.5056/jnm18194.
Toshihiko Tomita 1, Kazuki Fujimura 1, Haruki Asano 1, Tomohiro Ogawa 1, Takahisa Yamasaki 1, Takashi Kondo 1, Tomoaki Kono 1, Katsuyuki Tozawa 1, Tadayuki Oshima 1, Hirokazu Fukui 1, Takeshi Kimura 2, Jiro Watari 1, Hiroto Miwa 1
Affiliations
- PMID: 31587548
- PMCID: PMC6786451
- DOI: 10.5056/jnm18194
A Randomized Double-blind Placebo-controlled Trial on the Effect of Magnesium Oxide in Patients With Chronic Constipation
Sumire Mori et al. J Neurogastroenterol Motil. 2019.
Abstract
Background/aims: Magnesium oxide (MgO) has been frequently used as a treatment for chronic constipation (CC) since the 1980s in Japan. The aim of this study is to evaluate its therapeutic effects of MgO in Japanese CC patients.
Methods: We conducted a randomized, double-blind placebo-controlled study. Thirty-four female patients with mild to moderate constipation were randomly assigned to either placebo (n = 17) or MgO group (n = 17) 0.5 g × 3/day for 28 days. Primary endpoint was overall improvement over the 4-week study period. Secondary endpoints were changes from baseline in spontaneous bowel movement (SBM), response rates of complete spontaneous bowel movement (CSBM), stool form, colonic transit time (CTT), abdominal symptom, and quality of life.
Results: One patient failed to complete the medication regimen and was omitted from analysis: data from 16 placebo and 17 MgO patients were analyzed. The primary endpoint was met by 25.0% of placebo vs 70.6% of MgO group (P = 0.015). MgO significantly improved SBM changes compared to placebo ( P = 0.002). However, MgO did not significantly improved response rates of CSBM compared to placebo (P = 0.76). In addition, MgO significantly improved Bristol stool form scale changes (P < 0.001) and significantly improved CTT compared to the placebo group (P < 0.001). MgO significantly improved the Japanese version of the patient assessment of constipation quality of life (P = 0.003).
Conclusion: Our placebo-controlled study demonstrated that MgO was effective treatment for improving defecation status and shortened CTT in Japanese CC patients with mild to moderate symptoms.
Keywords: Constipation; Defecation; Double-blind method; Japan; Magnesium oxide.
Conflict of interest statement
Conflicts of interest: None.
Figures
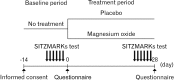
Figure 1
Study design.
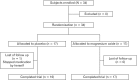
Figure 2
Patient’s flowchart summary.
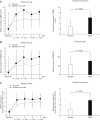
Figure 3
Effect of overall symptomatic improvement in Japanese patients with chronic constipation. (A) Weekly average value of overall improvement score. (B) Response rate of overall improvement. Compared to the response rate of 25.0% for overall symptom improvement in the placebo group, that with magnesium oxide (MgO) was significantly higher at 70.6%.
Figure 4
Changes of spontaneous bowel movement (SBM) and Bristol stool form scale (BSFS) after magnesium oxide (MgO) treatment in Japanese patients in chronic constipation. (A) Weekly average value of SBM and its change from baseline. (B) Weekly average value of complete spontaneous bowel movement (CSBM) and the response rate of CSBM. (C) Weekly average of BSFS score and its change from baseline.
Figure 5
Effect of magnesium oxide (MgO) on gastrointestinal symptoms in Japanese patients with chronic constipation. (C) The severity score for straining during defecation in the placebo group did not differ before versus after taking the drug, although there was significant improvement in the MgO group (P = 0.003). The 2 groups did not show any statistically significant differences in terms of (A) abdominal bloating, (B) abdominal discomfort, or (D) sensation of incomplete evacuation.
Figure 6
Change of quality of life ([A] short form-8 [SF-8] and the [B] Japanese version of the patient assessment of constipation quality of life [JPACQOL]) in Japanese patients with chronic constipation. The 2 groups also showed no differences in physical component summary (PCS) score and mental component summary (MCS) score. JPAC-QOL evaluations indicated that the degree of change in the total score before versus after the intervention was significantly higher in the magnesium oxide (MgO) group than in the placebo group.
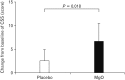
Figure 7
Change in the constipation scoring system (CSS) score in Japanese patients with chronic constipation. While administration of magnesium oxide (MgO) significantly improved defecation status, we did not observe any improvement in the placebo group (P = 0.018).
References
LinkOut - more resources
Full Text Sources
Miscellaneous