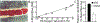Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle - PubMed (original) (raw)
Review
Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle
Richard T Premont et al. Circ Res. 2020.
Abstract
A continuous supply of oxygen is essential for the survival of multicellular organisms. The understanding of how this supply is regulated in the microvasculature has evolved from viewing erythrocytes (red blood cells [RBCs]) as passive carriers of oxygen to recognizing the complex interplay between Hb (hemoglobin) and oxygen, carbon dioxide, and nitric oxide-the three-gas respiratory cycle-that insures adequate oxygen and nutrient delivery to meet local metabolic demand. In this context, it is blood flow and not blood oxygen content that is the main driver of tissue oxygenation by RBCs. Herein, we review the lines of experimentation that led to this understanding of RBC function; from the foundational understanding of allosteric regulation of oxygen binding in Hb in the stereochemical model of Perutz, to blood flow autoregulation (hypoxic vasodilation governing oxygen delivery) observed by Guyton, to current understanding that centers on S-nitrosylation of Hb (ie, S-nitrosohemoglobin; SNO-Hb) as a purveyor of oxygen-dependent vasodilatory activity. Notably, hypoxic vasodilation is recapitulated by native S-nitrosothiol (SNO)-replete RBCs and by SNO-Hb itself, whereby SNO is released from Hb and RBCs during deoxygenation, in proportion to the degree of Hb deoxygenation, to regulate vessels directly. In addition, we discuss how dysregulation of this system through genetic mutation in Hb or through disease is a common factor in oxygenation pathologies resulting from microcirculatory impairment, including sickle cell disease, ischemic heart disease, and heart failure. We then conclude by identifying potential therapeutic interventions to correct deficits in RBC-mediated vasodilation to improve oxygen delivery-steps toward effective microvasculature-targeted therapies. To the extent that diseases of the heart, lungs, and blood are associated with impaired tissue oxygenation, the development of new therapies based on the three-gas respiratory system have the potential to improve the well-being of millions of patients.
Keywords: S-nitrosothiols; erythrocytes; heart diseases; nitric oxide; vasodilation.
Conflict of interest statement
DISCLOSURES
Drs. Reynolds and Stamler hold patents related to re-nitrosylation of blood, some of which have been licensed for commercial development. CWRU and UH are aware of these conflicts and appropriate management plans are in place. Drs. Premont and Zhang do not have any relevant conflicts to disclose.
Figures
Figure 1.. Tissue blood flow is controlled by hypoxic autoregulation during arterial-venous transit.
A. RBCs traversing microvascular capillaries in single-file requires RBC deformation due to intimate contact of RBC and endothelial cell membranes. RBCs adjoining the endothelium constitute an integrated vascular unit. B. Blood flow through the canine hind limb increases linearly as arterial blood oxygenation decreases, demonstrating that hypoxic vasodilation to maintain oxygen delivery is directly and effectively coupled to HbO2 saturation, not to PO2 or P50. C. Reactive hyperemia of the gastrocnemius muscle (shown as % over basal flow) in mice bearing human wildtype hemoglobin (C93), or where β-globin is mutated (C93A) and cannot bear SNO at this site. Modified panel A from Ref, panel B from Ref, panel C from Ref, with permission.
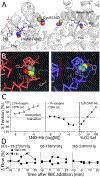
Figure 2.. Oxygen-driven conformational changes in SNO-βCys93-Hb allow SNO release.
A. Crystal structure of nitrosylated R-state hemoglobin. The β-globin hemes (with iron-coordinating His63 and His87 residues and heme-stabilizing Phe42 residue) and the reactive βCys93 residues, are NO bound. Cys sulfur atom, yellow; NO nitrogen atom, blue; NO oxygen atom, red; hemes shown as ball-and-stick, and β-globin backbone as ribbon. Derived from 1buw structure in Ref using PyMol. Note: SNO is released from Hb in the T-state, preventing crystallization. B. Model comparing SNO-Hb βCys93 in the oxygenated R-state (red backbone) with SNO buried (left), with deoxygenated T-state (blue backbone) with SNO exposed for NO bioactivity release (right). Cys sulfur atom, green; NO nitrogen atom, blue; NO oxygen atom, red. C. Isolated SNO-Hb (2SNO-Hb[FeO2]4) in the oxygenated state (21% O2; R-state) (left) induces contraction of blood vessels (aortic rings) through scavenging of NO derived from endothelium, but SNO-Hb in the deoxygenated state (<1% O2; T-state) (center) induces relaxation through release of SNO. Hb Cys-SNO content and O2 saturation are linked and linearly related to vessel relaxation activity (right), grey points extrapolated according to Refs., D. Blood flow through the substantia nigra of rat brain is dependent on SNO-Hb and blood oxygenation (at the indicated measured tissue PO2). SNO-repleted (circles) or native (squares) Hb was infused in femoral vein over 3 min starting at time 0. SNO-Hb mediated vasodilation is allosterically regulated by O2 release from Hb and thus proportional to tissue hypoxia. Modified panels B and D from Ref, panel C from Refs,, with permission.
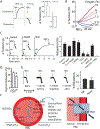
Figure 3.. RBC vasodilation through SNO-Hb.
A. Regulation of aortic ring tension after addition of native human RBCs (A-C) in high or low oxygen (Hb in R- vs T-state). RBCs pretreated with physiological amounts of NO (1μM) to produce ~500 nM SNO-Hb; representative traces (left) and quantification (right). RBCs recapitulate effects of native SNO-Hb (see Figure 2B). B. Graded relaxation by native untreated RBCs as a function of PO2 ranging from ~10% O2 (63 mmHg; HbO2 ~90% saturated) to ~0.5% O2 (3 mmHg; HbO2 <10%) across the R-T transition. C. Hypoxic (~1% O2; Hb T-state) vasodilation of aortic rings by native (untreated) human RBCs, after denuding (“D”) endothelial cells, after NOS inhibition by L-NAME, after cGC inhibition by ODQ, or in aorta from eNOS-knockout mice. Representative traces (left) and quantification (right). D. Hypoxic (~1% O2) vasodilation of aortic rings by mouse RBCs bearing human wildtype α,β,γ globins (C93), or where human β-globin is mutated (C93A) and cannot bear SNO at this site. Note that mouse RBCs have a predominant ATP-dependent vasorelaxation pathway (compared to human RBCs) that contributes to the residual activity in endothelium-intact vessels, and these mice carry residual C93 in fetal Hb. E. Hypoxic (~1% O2) vasodilation of aorta by native human RBCs is not altered by addition of micromolar nitrite, before, during or after addition of RBCs. Representative traces (left) and quantification (right). F. Model of PO2-driven SNO flux in and out of RBCs. SNO-Hb is formed de novo under high PO2. Under low PO2 in tissues, Hb conformation change allows SNO-Cys93 to transfer SNO to the transmembrane transporter AE1 (Cys 317), which shuttles SNO between coupled Cys residues to expose SNO on the outer membrane of the RBC, where it will equilibrate with SNO carriers in the blood and with SNO proteins in the vessel endothelial wall to elicit vessel dilation. Under high PO2, the AE1–Hb SNO equilibrium is proposed to run in reverse to return SNO into the RBC and onto Hb. Note; whereas SNO-Cys93 and SNOCys317 have been identified in NO export, involvement of Cys843 is proposed based on exchange of selenium between Cys317/Cys843 in studies on selenium import from plasma-to-Cys317. Modified panel A from Ref, panel B from Ref, panels C and E from Ref, and panel D from Ref, with permission.
Figure 4.. Human arterial-venous gradient in RBC SNO-Hb results from deoxyHb release of SNO that can be acquired by glutathione (extracellular thiol acceptor).
A. Arterial and venous blood O2 and SNO-Hb values from healthy humans. B. SNO-Hb (purified preparation; 2SNO-Hb[FeO2]; metHb <5%) levels decline upon deoxygenation, and SNO loss is accelerated markedly by addition of glutathione (GSH) as SNO acceptor. C. Linear relationship between Hb oxygen saturation and SNO-Hb (expressed as ln(SNO-Hb/Hb) in native human RBCs: SNO-Hb declines in RBCs with oxygen desaturation, effectively creating the gradient between artery and vein (see Figure 4A and 4D). Addition of GSH accelerates loss of Hb-SNO from RBCs reflecting equilibrium between SNO inside and outside RBCs. D. Export of SNO by native RBCs in vivo. GSH incubated with whole venous blood (blue, deoxy) acquires offloaded SNO (GSNO detected by LC/MS), while oxygenated (arterial) blood does not (red). Ultraviolet light-treatment to remove SNO from venous blood, control (purple). E. Aortic ring relaxation by native RBCs isolated from wildtype C93 mice is augmented by extracellular GSH, but augmentation is lost using RBCs from βC93A mutant mice. Modified panel A from Ref, panel B from Ref, panel C from Ref, panel D from Ref, panel E from Ref, with permission.
Figure 5.. Impact of βCys93 on cardiovascular and respiratory function.
A. Representative blood flow tracings during graded hypoxia in a γβC93 (wildtype human Hb) and a γβC93A (Cys mutant Hb refractory to S-nitrosylation) mouse. B. Muscle blood flow and C. PO2 responses to hypoxia are significantly compromised, with baseline blood flow and PO2 lower in the γβC93A and βC93A mutant mice. D. Representative ECG recordings in a C93 (control) and a C93A mouse at FiO2 of 0.21 and 0.05 with T- and ST-waves marked, indicating myocardial ischemia at baseline in the C93A mouse and myocardial infarction/injury with brief (5 min) hypoxic challenge. E. During transient progressive hypoxia, ST-wave elevation (and hyperacute T-waves) indicative of acute ischemic injury are significantly greater and far more frequent in βC93A and γβC93A mice vs. γβC93 control mice. F. At FiO2 = 0.21 (room air), T-wave amplitude is significantly reduced in βC93A and γβC93A mice vs. γβC93 mice, indicative of ischemia under basal conditions. G. Post-ischemia necrotic area (white) in heart after induced myocardial infarction is greater in βC93A mice (right) compared to wildtype βC93 mice (left). H. Cardiac output after transaortic constriction pressure overload (TAC) is depressed in βC93A and γβC93A mice, indicative of heart failure due to microvascular impairment. I. Survival during chronic TAC pressure overload heart failure is reduced in βC93A and γβC93A mice. J. Breathing frequency fails to increase in βC93A mutant mice after return to normoxia following hypoxic challenge indicating central respiratory impairment. K. Lung tidal volume fails to increase in βC93A mutant mice after return to normoxia following hypoxic challenge. L. Minute ventilation fails to increase in βC93A mutant mice after return to normoxia following hypoxic challenge. Modified panels A-F from Ref, panels G-I from Ref, panels J-L from Ref, with permission.
Figure 6.. Physiological and pathophysiological alterations in SNO-Hb.
A. Hb oxygen saturation (upper) and SNO-Hb levels (lower) measured in humans during staggered ascent to 5,000 meters and return to base altitude over 19 days. B. Levels of SNO-Hb and iron-nitrosyl Hb (HbFeNO), and thus total HbNO, are significantly reduced in patients with severe Pulmonary Arterial Hypertension (PAH), compared to age-matched controls. C. In PAH patients, NO/SNO levels inversely correlated with disease severity; specifically, higher pulmonary arterial pressures (PAP) were associated with lower RBC HbNO levels. D. Representative data showing that human RBCs dilate pulmonary artery in vitro in an NO-dependent manner (blocked by inhibition of sGC with ODQ) and that relaxations of aorta are impaired in RBCs from PAH patients. E. Group data demonstrating impaired ability of RBCs from PAH patients to produce hypoxic vasodilation in an in vitro aortic ring bioassay. F. SNO-Hb level is reduced in human sickle RBCs, and correlates with disease severity. G. Sickle RBCs are impaired by thiol oxidation of membrane transporter protein AE1 (Band 3), which is required for SNO export. H. Sickle RBCs from severe SCD patients are ineffective in hypoxic vasodilation in the in vitro aortic ring bioassay. I. The amount of NO bioactivity (ratio of SNO to total HbNO) is significantly less in blood from patients with Peripheral Artery Disease (PAD) compared to age-matched healthy controls. J. Representative tissue oxygenation (StO2) tracings depicting the delayed hyperemic response in a PAD patient after a brief period of arterial occlusion. K. Mean (± SD) foot reperfusion times following thigh occlusion are significantly longer in the PAD cohort. (* p< 0.05). Panel A modified from Ref, panels B-E from Ref, panels F,G,H from Ref, with permission; panels I,J,K, unpublished data.
Figure 7.. Blood storage diminishes SNO-Hb, hypoxic vasodilation and O2 delivery, and RBC SNO repletion improves tissue O2 delivery.
A. Storage of human-donor blood results in rapid loss of SNO-Hb. B. Loss of SNO-Hb correlates with diminished hypoxic vasodilation by RBCs. Aortic vasorelaxation by RBCs is restored by repleting SNO-Hb to physiological level (~1–5μM; see Figure 3A). C. Peripheral tissue oxygen saturation in mice declines following transfusion with stored blood (1–2 units equivalent), consistent with diminished hypoxic vasodilation and microvascular plugging (see Figure 1A, 1C). D. Canine coronary artery blood flow in vivo elicited by renitrosylated RBCs is significantly greater than that produced by SNO-depleted (stored) RBCs, with the degree of change greater under hypoxia. E. Time course of change in arterial-venous O2 content (mean ± SD) in sheep after replacement of 2 units of RBCs stored for 14 days (untreated, squares) or repleted with physiological amounts of SNO (1–5 μM) immediately prior to transfusion (circles, renitrosylated). *, higher than baseline, X, lower than baseline, p<0.05. F. Skeletal muscle PO2 in sheep transfused as in (E). *, higher than baseline, p<0.05. Modified panels A,B,D from Ref, Panel C from Ref and Panels E,F from Ref, with permission.
Similar articles
- Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via _β_Cys93.
Hausladen A, Qian Z, Zhang R, Premont RT, Stamler JS. Hausladen A, et al. J Pharmacol Exp Ther. 2022 Jul;382(1):1-10. doi: 10.1124/jpet.122.001194. Epub 2022 May 5. J Pharmacol Exp Ther. 2022. PMID: 35512801 Free PMC article. - Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Antioxid Redox Signal. 2021 Apr 20;34(12):936-961. doi: 10.1089/ars.2020.8153. Epub 2020 Jul 23. Antioxid Redox Signal. 2021. PMID: 32597195 Free PMC article. Review. - Essential Role of Hemoglobin βCys93 in Cardiovascular Physiology.
Premont RT, Stamler JS. Premont RT, et al. Physiology (Bethesda). 2020 Jul 1;35(4):234-243. doi: 10.1152/physiol.00040.2019. Physiology (Bethesda). 2020. PMID: 32490751 Free PMC article. Review. - SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation.
Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. Isbell TS, et al. Nat Med. 2008 Jul;14(7):773-7. doi: 10.1038/nm1771. Epub 2008 May 30. Nat Med. 2008. PMID: 18516054 Free PMC article. - How do red blood cells cause hypoxic vasodilation? The SNO-hemoglobin paradigm.
Allen BW, Piantadosi CA. Allen BW, et al. Am J Physiol Heart Circ Physiol. 2006 Oct;291(4):H1507-12. doi: 10.1152/ajpheart.00310.2006. Epub 2006 Jun 2. Am J Physiol Heart Circ Physiol. 2006. PMID: 16751292 Review.
Cited by
- Nitric oxide: Potential therapeutic target in Heat Stress-induced Multiple Organ Dysfunction.
Jaswal P, Bansal S, Chaudhary R, Basu J, Bansal N, Kumar S. Jaswal P, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Oct 28. doi: 10.1007/s00210-024-03556-z. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39466442 Review. - Protein S-Nitrosylation: A Chemical Modification with Ubiquitous Biological Activities.
Aboalroub AA, Al Azzam KM. Aboalroub AA, et al. Protein J. 2024 Aug;43(4):639-655. doi: 10.1007/s10930-024-10223-y. Epub 2024 Jul 28. Protein J. 2024. PMID: 39068633 Review. - The enzymatic function of the honorary enzyme: S-nitrosylation of hemoglobin in physiology and medicine.
Premont RT, Singel DJ, Stamler JS. Premont RT, et al. Mol Aspects Med. 2022 Apr;84:101056. doi: 10.1016/j.mam.2021.101056. Epub 2021 Nov 28. Mol Aspects Med. 2022. PMID: 34852941 Free PMC article. Review. - Post-Translational S-Nitrosylation of Proteins in Regulating Cardiac Oxidative Stress.
Shi X, Qiu H. Shi X, et al. Antioxidants (Basel). 2020 Oct 28;9(11):1051. doi: 10.3390/antiox9111051. Antioxidants (Basel). 2020. PMID: 33126514 Free PMC article. Review. - Neuronal nitric oxide synthase is required for erythropoietin stimulated erythropoiesis in mice.
Lee J, Dey S, Rajvanshi PK, Merling RK, Teng R, Rogers HM, Noguchi CT. Lee J, et al. Front Cell Dev Biol. 2023 Feb 21;11:1144110. doi: 10.3389/fcell.2023.1144110. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36895793 Free PMC article.
References
- Hoyert DL, Xu J. Deaths: Preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1–51 - PubMed
- Mensah GA, Brown DW. An overview of cardiovascular disease burden in the united states. Health Aff (Millwood). 2007;26:38–48 - PubMed
- Perutz MF. Relation between structure and sequence of haemoglobin. Nature. 1962;194:914–917 - PubMed
- Perutz MF. Mechanisms regulating the reactions of human hemoglobin with oxygen and carbon monoxide. Annu Rev Physiol. 1990;52:1–25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL075443/HL/NHLBI NIH HHS/United States
- P01 HL128192/HL/NHLBI NIH HHS/United States
- R01 DK119506/DK/NIDDK NIH HHS/United States
- R01 HL126900/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources