Impaired adult neurogenesis is an early event in Alzheimer's disease neurodegeneration, mediated by intracellular Aβ oligomers - PubMed (original) (raw)
. 2020 Mar;27(3):934-948.
doi: 10.1038/s41418-019-0409-3. Epub 2019 Oct 7.
Francesco Marrocco 3, Valentina Latina 2 4, Federica Ruggeri 2, Valerio Corvaglia 2 5, Federico La Regina 2, Martine Ammassari-Teule 6, Silvia Middei 3, Giuseppina Amadoro 2 4, Giovanni Meli 7, Raffaella Scardigli 8 9, Antonino Cattaneo 10 11
Affiliations
- PMID: 31591472
- PMCID: PMC7206128
- DOI: 10.1038/s41418-019-0409-3
Impaired adult neurogenesis is an early event in Alzheimer's disease neurodegeneration, mediated by intracellular Aβ oligomers
Chiara Scopa et al. Cell Death Differ. 2020 Mar.
Erratum in
- Correction to: Impaired adult neurogenesis is an early event in Alzheimer's disease neurodegeneration, mediated by intracellular Aβ oligomers.
Scopa C, Marrocco F, Latina V, Ruggeri F, Corvaglia V, La Regina F, Ammassari-Teule M, Middei S, Amadoro G, Meli G, Scardigli R, Cattaneo A. Scopa C, et al. Cell Death Differ. 2020 Jun;27(6):2035. doi: 10.1038/s41418-019-0478-3. Cell Death Differ. 2020. PMID: 31896795 Free PMC article.
Abstract
Alterations of adult neurogenesis have been reported in several Alzheimer's disease (AD) animal models and human brains, while defects in this process at presymptomatic/early stages of AD have not been explored yet. To address this, we investigated potential neurogenesis defects in Tg2576 transgenic mice at 1.5 months of age, a prodromal asymptomatic age in terms of Aβ accumulation and neurodegeneration. We observe that Tg2576 resident and SVZ-derived adult neural stem cells (aNSCs) proliferate significantly less. Further, they fail to terminally differentiate into mature neurons due to pathological, tau-mediated, and microtubule hyperstabilization. Olfactory bulb neurogenesis is also strongly reduced, confirming the neurogenic defect in vivo. We find that this phenotype depends on the formation and accumulation of intracellular A-beta oligomers (AβOs) in aNSCs. Indeed, impaired neurogenesis of Tg2576 progenitors is remarkably rescued both in vitro and in vivo by the expression of a conformation-specific anti-AβOs intrabody (scFvA13-KDEL), which selectively interferes with the intracellular generation of AβOs in the endoplasmic reticulum (ER). Altogether, our results demonstrate that SVZ neurogenesis is impaired already at a presymptomatic stage of AD and is caused by endogenously generated intracellular AβOs in the ER of aNSCs. From a translational point of view, impaired SVZ neurogenesis may represent a novel biomarker for AD early diagnosis, in association to other biomarkers. Further, this study validates intracellular Aβ oligomers as a promising therapeutic target and prospects anti-AβOs scFvA13-KDEL intrabody as an effective tool for AD treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Fig. 1
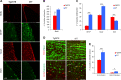
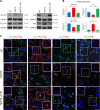
In vivo SVZ and OB neurogenesis is affected in Tg2576 mice. a Immunofluorescence staining for BrdU, progenitors, and neuroblasts markers in adult SVZ of Tg2576 and wild type (WT) mice. Immunostaining for incorporated BrdU in progenitor cells shows reduced proliferation in Tg2576 SVZ niche (red signal in top panels), while there is a significant reduction of aNSCs compartment (GFAP+ and Sox2+ cells) and an increase in DCX+ neuroblasts. Scale bar 100 µm, ×20 magnification (for BrdU) and 50 µm, ×40 magnification, zoom 1.3 (for SVZ markers). The histograms represent the quantification of BrdU (b) or Sox2, GFAP, and DCX positive cells (c) in Tg2576 (red) and WT (blue) SVZ. Data are means ± SEM of five individual animals (n = 5) for each experimental group. **P < 0.01, ***P < 0.001, significantly different from WT, Mann–Whitney test. d Immunostaining for BrdU and NeuN (red and green, respectively, top panels, ×40 magnification) and BrdU and Calretinin (red and green, respectively, bottom panels, ×63 magnification) shows a significant reduction of newborn Calretinin interneurons in Tg2576 olfactory bulbs compared with WT. Scale bar 50 µm. e Quantification of double positive cells in Tg2576 (red) and WT (blue). Data are means ± SEM of five individual animals (n = 5) for each experimental group. *P < 0.05, ***P < 0.001, significantly different from WT, Mann–Whitney test
Fig. 2
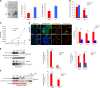
Proliferative and differentiation impairment of Tg2576 adult neural stem cells correlates with high levels of APP and AβOs. a Phase-contrast micrograph of aNSCs culture showing less primary neurospheres in Tg2576, that are also smaller in size, compared with WT. Scale bar 50 µm, ×10 magnification. Quantification of the number of primary neurospheres and of their size from single SVZ are expressed as mean ± SEM of five animals (n = 5) for each experimental group. *P < 0.05, significantly different from WT, Mann–Whitney test. b Quantification of Sox2 and DCX positive cells in SVZ-derived neurospheres from Tg2576 (red) and WT (blue) animals. In vitro analyses show a reduction of aNSCs compartment (Sox2+ cells) and a significant increase in DCX+ neuroblasts, confirming the in vivo data. Data are means ±SEM of five individual animals (n = 5) for each genotype. *P < 0.05, ***P < 0.001, significantly different from WT, Mann–Whitney test. c Cell proliferation is reduced in Tg2576 progenitors. Growth curves at early passages shows a significant decrease in proliferation of Tg2576 progenitors (red line) compared with WT sample (blue line). Quantification are expressed as mean ± SEM of two pools of five animals for each experimental group examined in three independent experiments (n = 6). **P < 0.01, significantly different from WT, Mann–Whitney test. d Aβ and APP are highly expressed in Tg2576 SVZ and SVZ-derived neurospheres, as revealed by immunofluorescence staining for Aβ42 in SVZ (anti-Aβ 12F4) and human Aβ/APP (anti-hAβ/hAPP D54D2). Double immunostaining for hAPP/hAβ (green signal) and progenitors or neuroblasts markers (red signal) shows hAPP/hAβ generation in both Sox2+ progenitors and DCX+ neuroblasts in Tg2576 but not in WT samples. Scale bar 50 µm, ×40 magnification, zoom 1.9 (for SVZ) and 10 µm, ×63 magnification, zoom 2.5 (for neurospheres). White squared boxes in top panels represent ×2 magnification of the corresponding dot-lines insets. DAPI staining on nuclei in blue. Quantification of immunofluorescence intensity of APP/Aβ-expressing cells (n = 50) in neurospheres (NS), progenitors, and neuroblasts, as mean ± SEM of the analysis of three independent experiments. **P < 0.01, significantly different from WT, Mann–Whitney test. e Western blot (anti-Aβ WO2) and dot blot (DB) analysis of Tg2576 (Tg1 and Tg2) and WT (WT1 and WT2) total lysates demonstrates that human APP, APP C-99, and AβOs are almost exclusively detected in Tg2576 samples, as quantified in the histograms on the right. Comparable results are obtained by western blot analysis of conditioned media (f), in which s-APP and Aβ monomers (4 KDa) are detectable only in Tg2576 media, as quantified in the as quantified in the histogram on the right. Quantifications are reported in comparison with WT samples (at 1), obtained from densitometric values of bands and spots normalized for β-actin (cell lysates), for one band of ponceau (conditioned media), or for the corresponding ponceau staining of the dot (DB). Data are mean ± SEM of two samples for each experimental group examined in three independent experiments (n = 6). *P < 0.05, ***P < 0.001 significantly different from WT, Student’s _t_-test
Fig. 3
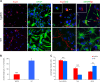
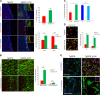
Differentiation impairment of Tg2576 aNSCs. a Immunostaining for TuJ1 (red) and GFAP (green) shows that Tg2576 progenitors fail to differentiate properly, as shown by the drastic reduction of neurites and the dystrophic cell shape of astrocytes. Both Tg2576 neurons and astrocytes express higher level of Aβ than their control counterpart (right panels, green and red signal, respectively, in the white squared boxes with the corresponding cell indicated by the arrows). Scale bar 50 μm, ×40 magnification, zoom 1.5 for the left panels and scale bar 20 μm, ×63 magnification, zoom 2 for the other panels. b Quantification of neurites length per number of neuron bodies in Tg2576 and WT samples is expressed as mean ± SEM of two samples for each experimental group examined in three independent experiments (n = 6). ***P < 0.001 significantly different from WT, Anova test. c Tg2576 differentiating neurospheres give rise to more TuJ1+ neurons at the expense of GFAP+ astrocytes. Quantification of percentage of positive TuJ1+ or GFAP+ cells in Tg2576 and WT samples is expressed as mean ± SEM of two samples for each exerimental groups examined in three independent experiments (n = 6). **P < 0.01 (for GFAP+ astrocytes), ***P < 0.001 (for TuJ1+ neurons) significantly different from WT, Mann–Whitney test. n.s., not significantly different from WT, Mann-Whitney test
Fig. 4
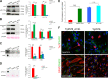
scFvA13-KDEL intrabody rescues the proliferative and differentiation impairment of Tg2576 aNSCs. a, b Western blot (WB) for full-length APP (anti-APP C-terminal) and for APP-CTFs, as well as for soluble APP (s-APP, anti-APP WO2) and soluble α-APP (sα-APP, anti-APP N-terminal 22C11) in Tg2576, WT and Tg2576_A13K lysates (a) and conditioned media (b) indicates that scFvA13 does not affect the processing of APP in the aNSCs, as quantified by the densitometric values reported in the corresponding histograms on the right. Dot Blot (DB) analysis on lysates (c) and conditioned media (d) shows that the stable expression of scFvA13-KDEL intrabody reduces the amount of AβOs to levels comparable to those measured in WT samples. The histograms shows the levels of Aβ species in WT, Tg2576 and Tg2576_A13K lysates and CM in comparison with Tg2576 samples (at 1), obtained from densitometric values of bands and normalized for β-actin (for lysates, a and c) or ponceau (for conditioned media, b and d). Data are mean ± SEM of two samples for each experimental group examined in three independent experiments (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from WT, Student’s _t_-test. n.s. not significantly different from Tg2576 or WT, Student's _t_-test. e Fold increase in cell number of aNSCs (Tg2576, Tg2576_A13K, WT, and WT_A13K) shows that the stable expression of scFvA13-KDEL intrabody leads to an undeniable rescue of the proliferative defect of Tg2576 progenitors, while does not influence proliferation of WT cells. Difference in proliferation rates has expressed as fold increase in cell number (FI), as mean ± SEM of two samples for each experimental group examined in three independent experiments (n = 6). **P < 0.01, significantly different from Tg2576, Mann–Whitney test. n.s., not significantly different from WT, Mann–Whitney test. f Immunofluorescence staining with D54D2 antibody for hAβ42/hAPP (green) and TuJ1 or GFAP (red) shows that in Tg2576_A13K differentiated cells there is a partial rescue of the dystrophic shape of astrocytes, while a more robust rescue of neurons maturation and arborization is evident, despite the Aβ production. Scale bar 25 μm, ×63 magnification, zoom 1.3 and 2.5 (neurons and astrocytes panels, respectively)
Fig. 5
Rescue of cytoskeleton alterations in Tg2576_A13K aNSCs. a Western blot analysis of Tg2576, Tg2576_A13K and WT cell lysates with antibodies against the Tau-1/AT8 epitopes and against acetyl (stable)- and tyrosinylated (instable)- α−tubulin. Tg2576 aNSCs display hyperstable microtubules, as indicated by the dramatic downregulation in AT8 Tau immunoreactivity (left panel) and the important upregulation in acetyl tubulin-positive microtubules (right panel). Interestingly, stable expression of scFvA13 intrabody is able to significantly rescue the cytoskeleton alteration, normalizing the microtubule instability to physiological control levels. b Densitometric quantification of the AT8, Tau1, acetyl-α−tubulin, and tyrosinylated-α−tubulin intensity bands in WT (blue), Tg2576 (red), and Tg2576_A13K (green) samples was calculated normalizing for GAPDH used as loading internal control. Data are means ± SEM of two samples for each experimental group examined in three independent experiments (n = 6). *P < 0.05, **P < 0.01, significantly different from WT, Student’s _t-_test. c Immunofluorescence staining for neurons (TuJ1, green signal) and the two forms of α−tubulin or Tau-1/AT8 epitopes (red signal) confirms the WB results. The higher level of acetyl-α−tubulin and of phosphorylated tau in Tg2576 samples are rescued at level comparable to those measured in WT by the stable expression of anti-AβOs intrabody. DAPI staining on nuclei in blue. Scale bar 25 μm, ×63 magnification, zoom 1.5. White squared boxes represent only the red signal of the corresponding dot-lines insets
Fig. 6
In vivo rescue of Tg2576 impaired neurogenesis by scFvA13-KDEL lentiviral infection. a Lentiviral delivery of scFvA13-KDEL intrabody to Tg2576 SVZ. The intrabody expression, confirmed by immunofluorescence staining for its C-terminal tag V5 (in green, right panels), leads to a significant rescue of proliferation, demonstrated by the increased number of BrdU+ cells (red signal), and restores the correct number of progenitors (Sox2+, red signal in central right panel) and neuroblasts (DCX+, red signal in bottom right panel) in the infected SVZ. DAPI staining on nuclei in blue. Scale bar 75 µm, ×40 magnification. White squared boxes in top panels represent ×2.5 magnification of the corresponding dot-lines insets. The histograms represent the quantification of BrdU or of Sox2 and DCX positive cells in Tg2576 (red) and Tg2576_A13K (green) SVZ. b Immunostaining for BrdU and NeuN (red and green, respectively, top panels) and BrdU and Calretinin (red and green, respectively, bottom panels) in the OB of infected animals shows a significant increase of newborn Calretinin interneurons derived from Tg2576_A13K SVZ progenitors compared with Tg2576, as quantified in the histogram. Scale bar 50 µm, ×40 magnification. c Quantification of the number of primary neurospheres derived from Tg2576 (red) and Tg2576_A13K (green) SVZ show ex-vivo rescue of proliferation by the in vivo intrabody expression. d Immunofluorescence staining for BrdU and Ki67 (red and green signals, respectively, in left panels), showing more BrdU+ (arrowheads) and double BrdU+Ki67+ (arrows) cells in Tg2576_A13K samples compared wth their noninfected counterpart. DAPI staining on nuclei in blue. Scale bar 25 µm, ×63 magnification. e Immunofluorescence staining for neurons (TuJ1, green signal in upper panels) and astrocytes (GFAP, green signal in lower panels) shows that the expression of scFvA13 intrabody (V5+ cells, red signal) leads to a partial rescue of both the neuronal arborization and the dystrophic cellular shape of astrocytes. DAPI staining on nuclei in blue. Scale bar 25 μm, ×63 magnification, zoom 1.5. White squared boxes represent only the V5 signal (in red) of the corresponding cells dot-lines insets. Data are means ± SEM of five individual animals (n = 5) for each experimental group. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from Tg2576, Mann-Whitney test. n.s., not significantly different from WT, Mann-Whitney test
Similar articles
- Conformational targeting of intracellular Aβ oligomers demonstrates their pathological oligomerization inside the endoplasmic reticulum.
Meli G, Lecci A, Manca A, Krako N, Albertini V, Benussi L, Ghidoni R, Cattaneo A. Meli G, et al. Nat Commun. 2014 May 27;5:3867. doi: 10.1038/ncomms4867. Nat Commun. 2014. PMID: 24861166 Free PMC article. - Characterization of age- and stage-dependent impaired adult subventricular neurogenesis in 5XFAD mouse model of Alzheimer's disease.
Park HH, Kim BH, Leem SH, Park YH, Hoe HS, Nam Y, Kim S, Shin SJ, Moon M. Park HH, et al. BMB Rep. 2023 Sep;56(9):520-525. doi: 10.5483/BMBRep.2023-0071. BMB Rep. 2023. PMID: 37482752 Free PMC article. - APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.
Crews L, Rockenstein E, Masliah E. Crews L, et al. Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review. - Early in vivo Effects of the Human Mutant Amyloid-β Protein Precursor (hAβPPSwInd) on the Mouse Olfactory Bulb.
Rusznák Z, Kim WS, Hsiao JH, Halliday GM, Paxinos G, Fu Y. Rusznák Z, et al. J Alzheimers Dis. 2016;49(2):443-57. doi: 10.3233/JAD-150368. J Alzheimers Dis. 2016. PMID: 26484907 - Promoting Endogenous Neurogenesis as a Treatment for Alzheimer's Disease.
Zhang Q, Liu J, Chen L, Zhang M. Zhang Q, et al. Mol Neurobiol. 2023 Mar;60(3):1353-1368. doi: 10.1007/s12035-022-03145-2. Epub 2022 Nov 29. Mol Neurobiol. 2023. PMID: 36445633 Review.
Cited by
- Beyond anosmia: olfactory dysfunction as a common denominator in neurodegenerative and neurodevelopmental disorders.
Chen YN, Kostka JK. Chen YN, et al. Front Neurosci. 2024 Oct 30;18:1502779. doi: 10.3389/fnins.2024.1502779. eCollection 2024. Front Neurosci. 2024. PMID: 39539496 Free PMC article. Review. - Transcriptomic analysis of CNTF-treated mouse subventricular zone-derived neurosphere culture reveals key transcription factor genes related to adult neurogenesis.
Kathanadan Chackochan B, Johnson S, Thameemul Ansari HJ, Vengellur A, Sivan U, Koyyappurath S, P S BC. Kathanadan Chackochan B, et al. Heliyon. 2024 Sep 28;10(19):e38496. doi: 10.1016/j.heliyon.2024.e38496. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39430537 Free PMC article. - The intricate interplay between microglia and adult neurogenesis in Alzheimer's disease.
Früholz I, Meyer-Luehmann M. Früholz I, et al. Front Cell Neurosci. 2024 Sep 18;18:1456253. doi: 10.3389/fncel.2024.1456253. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39360265 Free PMC article. Review. - Melatonin Augments the Expression of Core Transcription Factors in Aged and Alzheimer's Patient Skin Fibroblasts.
Shukla M, Duangrat R, Nopparat C, Sotthibundhu A, Govitrapong P. Shukla M, et al. Biology (Basel). 2024 Sep 5;13(9):698. doi: 10.3390/biology13090698. Biology (Basel). 2024. PMID: 39336125 Free PMC article. - Comprehensive characterization of the neurogenic and neuroprotective action of a novel TrkB agonist using mouse and human stem cell models of Alzheimer's disease.
Charou D, Rogdakis T, Latorrata A, Valcarcel M, Papadogiannis V, Athanasiou C, Tsengenes A, Papadopoulou MA, Lypitkas D, Lavigne MD, Katsila T, Wade RC, Cader MZ, Calogeropoulou T, Gravanis A, Charalampopoulos I. Charou D, et al. Stem Cell Res Ther. 2024 Jul 6;15(1):200. doi: 10.1186/s13287-024-03818-w. Stem Cell Res Ther. 2024. PMID: 38971770 Free PMC article.
References
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases