The Use of Test-negative Controls to Monitor Vaccine Effectiveness: A Systematic Review of Methodology - PubMed (original) (raw)
The Use of Test-negative Controls to Monitor Vaccine Effectiveness: A Systematic Review of Methodology
Huiying Chua et al. Epidemiology. 2020 Jan.
Abstract
Background: The test-negative design is an increasingly popular approach for estimating vaccine effectiveness (VE) due to its efficiency. This review aims to examine published test-negative design studies of VE and to explore similarities and differences in methodological choices for different diseases and vaccines.
Methods: We conducted a systematic search on PubMed, Web of Science, and Medline, for studies reporting the effectiveness of any vaccines using a test-negative design. We screened titles and abstracts and reviewed full texts to identify relevant articles. We created a standardized form for each included article to extract information on the pathogen of interest, vaccine(s) being evaluated, study setting, clinical case definition, choices of cases and controls, and statistical approaches used to estimate VE.
Results: We identified a total of 348 articles, including studies on VE against influenza virus (n = 253), rotavirus (n = 48), pneumococcus (n = 24), and nine other pathogens. Clinical case definitions used to enroll patients were similar by pathogens of interest but the sets of symptoms that defined them varied substantially. Controls could be those testing negative for the pathogen of interest, those testing positive for nonvaccine type of the pathogen of interest, or a subset of those testing positive for alternative pathogens. Most studies controlled for age, calendar time, and comorbidities.
Conclusions: Our review highlights similarities and differences in the application of the test-negative design that deserve further examination. If vaccination reduces disease severity in breakthrough infections, particular care must be taken in interpreting vaccine effectiveness estimates from test-negative design studies.
Conflict of interest statement
POTENTIAL CONFLICTS OF INTEREST
BJC has received honoraria from Sanofi Pasteur and Roche. The authors report no other potential conflicts of interest.
Figures
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the process and results of study screening.
Figure 2.
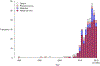
Number of included studies by year. Most of the studies identified were of influenza virus. The first eligible study was published in 1980 (16).
Figure 3.
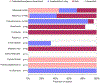
Study setting by pathogen. Patients could be recruited from outpatient/emergency department, inpatient, or both outpatient and inpatient setting.
Figure 4.
Choices of clinical case definition by pathogen. Studies that reported recruitment of patients meeting certain clinical case criteria without clarifying specific symptoms were excluded from this figure, including 32 influenza studies (recruited influenza-like illness patients), seven rotavirus studies (recruited gastroenteritis patients), two poliovirus studies (recruited acute flaccid paralysis patients), and one B. pertussis study (recruited pertussis-like-illness patients). Human papillomavirus and N. gonorrhoeae studies which recruited patients from routine testing were also excluded. Panel A: Other respiratory symptoms include wheezing, whooping cough, apnea, dyspnea, shortness of breath, bronchitis, pharyngitis, pneumonia etc. Other symptoms include complications such as sepsis, stroke, acute exacerbations of chronic respiratory conditions, contact history, etc, or for the case of measles virus, rash, dermal eruption etc. Panel B: Clinical case definition by culture-positive include eight studies which recruited patients with invasive pneumococcal disease and one with acute otitis media. DNA detection may include polymerase chain reaction, or multilocus sequence typing wherever specified. Biochemical tests include bile solubility and optochin susceptibility test. Panel D: Other symptoms include neck stiffness, altered consciousness, other meningeal signs.
Figure 5.
Choices of cases and controls by pathogen. Purple indicates cases and light green indicates controls. If patient samples were tested-positive for pathogen of interest, patient samples may be further tested to identify whether they were infected by vaccine-type or non-vaccine-type. If patients’ samples tested negative for the pathogen of interest, samples may be further tested. Alternative pathogens may be identified or patient samples may be undiagnosed or pan-negative (i.e. negative for all tested pathogen). Studies can be counted more than once when there was more than one choice of cases or controls.
Figure 6.
Proportion of studies that included age, sex, calendar time, or comorbidities in statistical model to estimate VE.
Similar articles
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - The case test-negative design for studies of the effectiveness of influenza vaccine.
Foppa IM, Haber M, Ferdinands JM, Shay DK. Foppa IM, et al. Vaccine. 2013 Jun 26;31(30):3104-9. doi: 10.1016/j.vaccine.2013.04.026. Epub 2013 Apr 23. Vaccine. 2013. PMID: 23624093 - Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies.
Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ. Belongia EA, et al. Lancet Infect Dis. 2016 Aug;16(8):942-51. doi: 10.1016/S1473-3099(16)00129-8. Epub 2016 Apr 6. Lancet Infect Dis. 2016. PMID: 27061888 Review. - A comparison of the test-negative and the traditional case-control study designs for estimation of influenza vaccine effectiveness under nonrandom vaccination.
Shi M, An Q, Ainslie KEC, Haber M, Orenstein WA. Shi M, et al. BMC Infect Dis. 2017 Dec 8;17(1):757. doi: 10.1186/s12879-017-2838-2. BMC Infect Dis. 2017. PMID: 29216845 Free PMC article. - Vaccines for preventing influenza in the elderly.
Rivetti D, Jefferson T, Thomas R, Rudin M, Rivetti A, Di Pietrantonj C, Demicheli V. Rivetti D, et al. Cochrane Database Syst Rev. 2006 Jul 19;(3):CD004876. doi: 10.1002/14651858.CD004876.pub2. Cochrane Database Syst Rev. 2006. PMID: 16856068 Updated. Review.
Cited by
- Influenza Vaccine Effectiveness and Progress Towards a Universal Influenza Vaccine.
Cowling BJ, Okoli GN. Cowling BJ, et al. Drugs. 2024 Sep;84(9):1013-1023. doi: 10.1007/s40265-024-02083-8. Epub 2024 Aug 21. Drugs. 2024. PMID: 39167316 Free PMC article. Review. - Incorporating Real-time Influenza Detection Into the Test-negative Design for Estimating Influenza Vaccine Effectiveness: The Real-time Test-negative Design (rtTND).
Feldstein LR, Self WH, Ferdinands JM, Randolph AG, Aboodi M, Baughman AH, Brown SM, Exline MC, Files DC, Gibbs K, Ginde AA, Gong MN, Grijalva CG, Halasa N, Khan A, Lindsell CJ, Newhams M, Peltan ID, Prekker ME, Rice TW, Shapiro NI, Steingrub J, Talbot HK, Halloran ME, Patel M; Influenza Vaccine Effectiveness in the Critically Ill (IVY) Investigators and the Pediatric Intensive Care Influenza Vaccine Effectiveness (PICFLU-VE) Investigators. Feldstein LR, et al. Clin Infect Dis. 2021 May 4;72(9):1669-1675. doi: 10.1093/cid/ciaa1453. Clin Infect Dis. 2021. PMID: 32974644 Free PMC article. - Use of Recently Vaccinated Individuals to Detect Bias in Test-Negative Case-Control Studies of COVID-19 Vaccine Effectiveness.
Hitchings MDT, Lewnard JA, Dean NE, Ko AI, Ranzani OT, Andrews JR, Cummings DAT. Hitchings MDT, et al. Epidemiology. 2022 Jul 1;33(4):450-456. doi: 10.1097/EDE.0000000000001484. Epub 2022 Apr 1. Epidemiology. 2022. PMID: 35384900 Free PMC article. - Double Negative Control Inference in Test-Negative Design Studies of Vaccine Effectiveness.
Li KQ, Shi X, Miao W, Tchetgen ET. Li KQ, et al. J Am Stat Assoc. 2024;119(547):1859-1870. doi: 10.1080/01621459.2023.2220935. Epub 2023 Jul 10. J Am Stat Assoc. 2024. PMID: 39524693 Free PMC article. - Vaccine effectiveness of primary series and booster doses against covid-19 associated hospital admissions in the United States: living test negative design study.
Adams K, Rhoads JP, Surie D, Gaglani M, Ginde AA, McNeal T, Talbot HK, Casey JD, Zepeski A, Shapiro NI, Gibbs KW, Files DC, Hager DN, Frosch AE, Exline MC, Mohamed A, Johnson NJ, Steingrub JS, Peltan ID, Brown SM, Martin ET, Lauring AS, Khan A, Busse LW, Duggal A, Wilson JG, Chang SY, Mallow C, Kwon JH, Chappell JD, Halasa N, Grijalva CG, Lindsell CJ, Lester SN, Thornburg NJ, Park S, McMorrow ML, Patel MM, Tenforde MW, Self WH; Influenza and other Viruses in the AcutelY ill (IVY) Network. Adams K, et al. BMJ. 2022 Oct 11;379:e072065. doi: 10.1136/bmj-2022-072065. BMJ. 2022. PMID: 36220174 Free PMC article.
References
- Smith PG, Rodrigues LC, Fine PE. Assessment of the protective efficacy of vaccines against common diseases using case–control and cohort studies. Int J Epidemiol. 1984;13(1):87–93. - PubMed
- Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case–control studies. I. Principles. Am J Epidemiol. 1992;135(9):1019–28. - PubMed