The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms - PubMed (original) (raw)
Review
The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms
Natali L Chanaday et al. J Neurosci. 2019.
Abstract
Neurotransmission is sustained by endocytosis and refilling of synaptic vesicles (SVs) locally within the presynapse. Until recently, a consensus formed that after exocytosis, SVs are recovered by either fusion pore closure (kiss-and-run) or clathrin-mediated endocytosis directly from the plasma membrane. However, recent data have revealed that SV formation is more complex than previously envisaged. For example, two additional recycling pathways have been discovered, ultrafast endocytosis and activity-dependent bulk endocytosis, in which SVs are regenerated from the internalized membrane and synaptic endosomes. Furthermore, these diverse modes of endocytosis appear to influence both the molecular composition and subsequent physiological role of individual SVs. In addition, previously unknown complexity in SV refilling and reclustering has been revealed. This review presents a modern view of the SV life cycle and discusses how neuronal subtype, physiological temperature, and individual activity patterns can recruit different endocytic modes to generate new SVs and sculpt subsequent presynaptic performance.
Copyright © 2019 the authors.
Figures
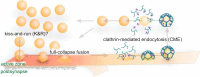
Figure 1.
Classical view of the SV cycle. Action potentials trigger fusion of SVs at the active zone. After formation of the fusion pore, resulting in neurotransmitter release, two options are possible: the pore can reclose via kiss-and-run (K&R) or it can expand irreversibly, leading to full collapse. Compensating for full-collapse fusion, specific SV proteins are recruited by adaptor proteins at the periactive zone, triggering CME. Dynamin mediates vesicle scission, after which SVs are uncoated and refilled with neurotransmitters before being returned to the vesicle cluster.
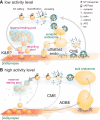
Figure 2.
Modern view of the SV cycle. A, During low activity levels, SVs are recruited to the RRP from the reserve/resting pool and fuse at the active zone, after which they may be retrieved via one of several mechanisms: (1) the fusion pore may reclose by kiss-and-run, (2) ultrafast endocytosis at the periactive zone can retrieve endocytic vesicles that rapidly fuse with synaptic endosomes from which SVs regenerate in a clathrin-dependent manner, or (3) CME can generate SVs from the plasma membrane in certain circumstances, after which vesicle uncoating is necessary for the vATPase to acidify the lumen triggering concurrent neurotransmitter (NT) refilling by transporters. After refilling, SVs can be recruited back to the cluster, where they are segregated into functional pools. B, At high activity levels, many SVs are mobilized and exocytosed by full-collapse fusion. This activates ADBE, which retrieves large areas of membrane generating bulk endosomes from which SVs regenerate.
Similar articles
- Synaptic Vesicle Endocytosis Occurs on Multiple Timescales and Is Mediated by Formin-Dependent Actin Assembly.
Soykan T, Kaempf N, Sakaba T, Vollweiter D, Goerdeler F, Puchkov D, Kononenko NL, Haucke V. Soykan T, et al. Neuron. 2017 Feb 22;93(4):854-866.e4. doi: 10.1016/j.neuron.2017.02.011. Neuron. 2017. PMID: 28231467 - Modes and mechanisms of synaptic vesicle recycling.
Soykan T, Maritzen T, Haucke V. Soykan T, et al. Curr Opin Neurobiol. 2016 Aug;39:17-23. doi: 10.1016/j.conb.2016.03.005. Epub 2016 Mar 24. Curr Opin Neurobiol. 2016. PMID: 27016897 Review. - A dynamin 1-, dynamin 3- and clathrin-independent pathway of synaptic vesicle recycling mediated by bulk endocytosis.
Wu Y, O'Toole ET, Girard M, Ritter B, Messa M, Liu X, McPherson PS, Ferguson SM, De Camilli P. Wu Y, et al. Elife. 2014 Jun 24;3:e01621. doi: 10.7554/eLife.01621. Elife. 2014. PMID: 24963135 Free PMC article. - Endosome-mediated endocytic mechanism replenishes the majority of synaptic vesicles at mature CNS synapses in an activity-dependent manner.
Park J, Cho OY, Kim JA, Chang S. Park J, et al. Sci Rep. 2016 Aug 18;6:31807. doi: 10.1038/srep31807. Sci Rep. 2016. PMID: 27534442 Free PMC article. - Synaptic vesicle generation from central nerve terminal endosomes.
Kokotos AC, Cousin MA. Kokotos AC, et al. Traffic. 2015 Mar;16(3):229-40. doi: 10.1111/tra.12235. Epub 2014 Nov 27. Traffic. 2015. PMID: 25346420 Review.
Cited by
- Neural Circuit Remodeling: Mechanistic Insights from Invertebrates.
Liu S, Alexander KD, Francis MM. Liu S, et al. J Dev Biol. 2024 Oct 11;12(4):27. doi: 10.3390/jdb12040027. J Dev Biol. 2024. PMID: 39449319 Free PMC article. Review. - Clathrin-associated carriers enable recycling through a kiss-and-run mechanism.
Xu J, Liang Y, Li N, Dang S, Jiang A, Liu Y, Guo Y, Yang X, Yuan Y, Zhang X, Yang Y, Du Y, Shi A, Liu X, Li D, He K. Xu J, et al. Nat Cell Biol. 2024 Oct;26(10):1652-1668. doi: 10.1038/s41556-024-01499-4. Epub 2024 Sep 19. Nat Cell Biol. 2024. PMID: 39300312 - Remodeling without destruction: non-proteolytic ubiquitin chains in neural function and brain disorders.
Zajicek A, Yao WD. Zajicek A, et al. Mol Psychiatry. 2021 Jan;26(1):247-264. doi: 10.1038/s41380-020-0849-7. Epub 2020 Jul 24. Mol Psychiatry. 2021. PMID: 32709994 Free PMC article. Review. - Local regulation of extracellular vesicle traffic by the synaptic endocytic machinery.
Blanchette CR, Scalera AL, Harris KP, Zhao Z, Dresselhaus EC, Koles K, Yeh A, Apiki JK, Stewart BA, Rodal AA. Blanchette CR, et al. J Cell Biol. 2022 May 2;221(5):e202112094. doi: 10.1083/jcb.202112094. Epub 2022 Mar 23. J Cell Biol. 2022. PMID: 35320349 Free PMC article. - Dynamin is primed at endocytic sites for ultrafast endocytosis.
Imoto Y, Raychaudhuri S, Ma Y, Fenske P, Sandoval E, Itoh K, Blumrich EM, Matsubayashi HT, Mamer L, Zarebidaki F, Söhl-Kielczynski B, Trimbuch T, Nayak S, Iwasa JH, Liu J, Wu B, Ha T, Inoue T, Jorgensen EM, Cousin MA, Rosenmund C, Watanabe S. Imoto Y, et al. Neuron. 2022 Sep 7;110(17):2815-2835.e13. doi: 10.1016/j.neuron.2022.06.010. Epub 2022 Jul 8. Neuron. 2022. PMID: 35809574 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- R01 MH066198/MH/NIMH NIH HHS/United States
- R01 NS105810/NS/NINDS NIH HHS/United States
- DP2 NS111133/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 NS078165/NS/NINDS NIH HHS/United States
- 204954/Z/16/Z /WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources