The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin - PubMed (original) (raw)
The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin
Maria Musarra-Pizzo et al. Nutrients. 2019.
Abstract
Due to their antimicrobial and antiviral activity potential in vitro, polyphenols are gaining a lot of attention from the pharmaceutical and healthcare industries. A novel antiviral and antimicrobial approach could be based on the use of polyphenols obtained from natural sources. Here, we tested the antibacterial and antiviral effect of a mix of polyphenols present in natural almond skin (NS MIX). The antimicrobial potential was evaluated against the standard American Type Culture Collection (ATCC) and clinical strains of Staphylococcus aureus, including methicillin-resistant (MRSA) strains, by minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). Herpes simplex virus type I was used for the antiviral assessment of NS MIX by plaque assay. Furthermore, we evaluated the expression of viral cascade antigens. NS MIX exhibited antimicrobial (MIC values of 0.31-1.25 mg/ml) and antiviral activity (decrease in the viral titer ** p < 0.01, and viral DNA accumulation * p < 0.05) against Staphylococcus aureus and HSV-1, respectively. Amongst the isolated compounds, the aglycones epicatechin and catechin showed the greatest activity against S. aureus ATCC 6538P (MIC values of 0.078-0.15 and 0.15 mg/ml, respectively), but were not active against all the other strains. These results could be used to develop novel products for topical use.
Keywords: S. aureus; almonds; antimicrobial; antiviral; herpes simplex virus; polyphenols.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures
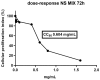
Figure 1
Viability of Vero cells treated with NS MIX. Vero cells were incubated with 1.6, 0.8, 0.6, 0.4, 0.2, and 0.1 mg/mL of NS MIX for 72 h. DMSO was used as a negative control. Cells were then collected and their viability was determined using the ViaLight™ plus cell proliferation and cytotoxicity bioassay kit (Lonza Group Ltd, Basel, Switzerland). The luminescence value was converted into cellular proliferation index as described in Materials and Methods. Results represent the mean of three biological independent experiments ± SD.
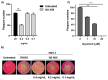
Figure 2
Antiviral activity of NS MIX. Vero cells were pre-treated with 0.4, 0.2, and 0.1 mg/mL of NS MIX for 1 h and the cells were then infected with Herpes simplex virus type 1 strain F (HSV-1) for 1 h at 37 °C under gentle shaking. After incubation, the inoculum was removed and the cells were covered with medium containing 0.8% methylcellulose in the presence of NS MIX at 0.4, 0.2, and 0.1 mg/mL, separately. After three days the cells were fixed and stained with crystal violet and the plaques were visualized with an inverted microscope. (A) Plaque reduction assay following the NS MIX treatment. (B) Plaque morphological change due to the NS MIX treatment. Results are the mean of three biological independent experiments ± SD for each dilution. (C) Antiviral activity of acyclovir was tested in Vero cells infected with HSV-1 and incubated with medium containing 0.8% methylcellulose in the presence of acyclovir at 1, 10, and 20 μM. The cells were stained with crystal violet for plaque counting and detection. Data are expressed as a mean (± SD) of at least three experiments and asterisks (**, ***) indicate the significance of _p_-values less than 0.01, and 0.001, respectively.
Figure 3
NS MIX affected the expression of viral antigens and HSV-1 replication. (A) Normal phase contrast inverted micrographs of Vero cells treated with 0.4 mg/mL of NS MIX. The cells were pre-treated with NS MIX for 1 h at 37 °C, then cells either mock infected or infected with HSV-1 at multiplicity of infection (MOI) of 1 for 1 h and incubated in presence of 0.4 mg/mL of the NS MIX for 24 h. (B) Immunoblot analysis was performed to detect α (ICP0), β (UL42), and γ (US11) viral proteins. GAPDH protein was used as loading control. Band density indicated in the figure was determined with the TINA program (version 2.10, Raytest, Straubenhardt, Germany) and expressed as the fold change over the housekeeping gene GAPDH. (C) Relative quantization of viral DNA was performed using real-time quantitative PCR and analyzed by the comparative Ct method (ΔΔCt). Data are expressed as a mean (± SD) of at least three experiments and asterisk (*) indicate the significance of _p_-values less than 0.05.
Similar articles
- Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell.
Bisignano C, Mandalari G, Smeriglio A, Trombetta D, Pizzo MM, Pennisi R, Sciortino MT. Bisignano C, et al. Viruses. 2017 Jul 10;9(7):178. doi: 10.3390/v9070178. Viruses. 2017. PMID: 28698509 Free PMC article. - Phytochemical Characterization of Olea europea Leaf Extracts and Assessment of Their Anti-Microbial and Anti-HSV-1 Activity.
Ben-Amor I, Musarra-Pizzo M, Smeriglio A, D'Arrigo M, Pennisi R, Attia H, Gargouri B, Trombetta D, Mandalari G, Sciortino MT. Ben-Amor I, et al. Viruses. 2021 Jun 7;13(6):1085. doi: 10.3390/v13061085. Viruses. 2021. PMID: 34200316 Free PMC article. - Anti-Herpes Simplex 1 Activity of Simmondsia chinensis (Jojoba) Wax.
Tietel Z, Melamed S, Eretz-Kdosha N, Guetta A, Gvirtz R, Ogen-Shtern N, Dag A, Cohen G. Tietel Z, et al. Molecules. 2021 Oct 7;26(19):6059. doi: 10.3390/molecules26196059. Molecules. 2021. PMID: 34641603 Free PMC article. - Polyphenols as antimicrobial agents.
Daglia M. Daglia M. Curr Opin Biotechnol. 2012 Apr;23(2):174-81. doi: 10.1016/j.copbio.2011.08.007. Epub 2011 Sep 16. Curr Opin Biotechnol. 2012. PMID: 21925860 Review. - Pistacia vera L. as natural source against antimicrobial and antiviral resistance.
Mandalari G, Pennisi R, Gervasi T, Sciortino MT. Mandalari G, et al. Front Microbiol. 2024 Jul 1;15:1396514. doi: 10.3389/fmicb.2024.1396514. eCollection 2024. Front Microbiol. 2024. PMID: 39011148 Free PMC article. Review.
Cited by
- Composition of Nuts and Their Potential Health Benefits-An Overview.
Gonçalves B, Pinto T, Aires A, Morais MC, Bacelar E, Anjos R, Ferreira-Cardoso J, Oliveira I, Vilela A, Cosme F. Gonçalves B, et al. Foods. 2023 Feb 23;12(5):942. doi: 10.3390/foods12050942. Foods. 2023. PMID: 36900459 Free PMC article. Review. - Use of Almond Skins to Improve Nutritional and Functional Properties of Biscuits: An Example of Upcycling.
Pasqualone A, Laddomada B, Boukid F, Angelis D, Summo C. Pasqualone A, et al. Foods. 2020 Nov 20;9(11):1705. doi: 10.3390/foods9111705. Foods. 2020. PMID: 33233841 Free PMC article. - Specific Antimicrobial Activities Revealed by Comparative Evaluation of Selected Gemmotherapy Extracts.
Héjja M, Mihok E, Alaya A, Jolji M, György É, Meszaros N, Turcus V, Oláh NK, Máthé E. Héjja M, et al. Antibiotics (Basel). 2024 Feb 13;13(2):181. doi: 10.3390/antibiotics13020181. Antibiotics (Basel). 2024. PMID: 38391567 Free PMC article. - Polyphenols: Secondary Metabolites with a Biological Impression.
Bolat E, Sarıtaş S, Duman H, Eker F, Akdaşçi E, Karav S, Witkowska AM. Bolat E, et al. Nutrients. 2024 Aug 3;16(15):2550. doi: 10.3390/nu16152550. Nutrients. 2024. PMID: 39125431 Free PMC article. Review. - Valorization of Almond (Prunus serotina) by Obtaining Bioactive Compounds.
Gallardo-Rivera CT, Lu A, Treviño-Garza MZ, García-Márquez E, Amaya-Guerra C, Aguilera C, Báez-González JG. Gallardo-Rivera CT, et al. Front Nutr. 2021 May 31;8:663953. doi: 10.3389/fnut.2021.663953. eCollection 2021. Front Nutr. 2021. PMID: 34136520 Free PMC article.
References
- Haider S., Batool Z., Haleem D.J. Nootropic and hyphofagic effects following long term intake of almonds (Prunus amygdalus) in rats. Nutr. Hosp. 2012;27:2109–2115. - PubMed
- U.S. Food and Drug Administration Qualified Health Claims: Letter of Enforcement Discretion-Nuts and Coronary Heart Disease (Docket No. 02P-0505) [(accessed on 1 September 2019)];2003 Jul 14; Office of Nutritional Products, Labeling and Dietary Supplements. Available online: http://www.cfsan.fda.gov/~dms/qhcnuts2.html.
- Mandalari G., Tomaino A., Arcoraci T., Martorana M., Lo Turco V., Cacciola F., Rich G.T., Bisignano C., Saija A., Dugo P., et al. Characterization of polyphenols, lipids and dietary fibre from skins of almonds (Amygdalus communis L.) J. Food Comp. Anal. 2010;23:166–174. doi: 10.1016/j.jfca.2009.08.015. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous