A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer - PubMed (original) (raw)
Review
. 2019 Oct 22;11(10):1618.
doi: 10.3390/cancers11101618.
Mihail Buse 2, Constantin Busuioc 3, Rares Drula 4, Diana Gulei 5, Lajos Raduly 4, Alexandru Rusu 6, Alexandru Irimie [ 7](#full-view-affiliation-7 "Department of Surgery, The Oncology Institute "Prof. Dr. Ion Chiricuta", 40015 Cluj-Napoca, Romania. airimie@umfcluj.ro.") 8, Atanas G Atanasov 9 10 11, Ondrej Slaby 12 13, Calin Ionescu 14 15, Ioana Berindan-Neagoe 16 17 18
Affiliations
- PMID: 31652660
- PMCID: PMC6827047
- DOI: 10.3390/cancers11101618
Review
A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer
Cornelia Braicu et al. Cancers (Basel). 2019.
Abstract
The mitogen-activated protein kinase (MAPK) pathway is an important bridge in the switch from extracellular signals to intracellular responses. Alterations of signaling cascades are found in various diseases, including cancer, as a result of genetic and epigenetic changes. Numerous studies focused on both the homeostatic and the pathologic conduct of MAPK signaling; however, there is still much to be deciphered in terms of regulation and action models in both preclinical and clinical research. MAPK has implications in the response to cancer therapy, particularly the activation of the compensatory pathways in response to experimental MAPK inhibition. The present paper discusses new insights into MAPK as a complex cell signaling pathway with roles in the sustenance of cellular normal conduit, response to cancer therapy, and activation of compensatory pathways. Unfortunately, most MAPK inhibitors trigger resistance due to the activation of compensatory feed-back loops in tumor cells and tumor microenvironment components. Therefore, novel combinatorial therapies have to be implemented for cancer management in order to restrict the possibility of alternative pathway activation, as a perspective for developing novel therapies based on integration in translational studies.
Keywords: MAPK; cancer; drug resistance; molecular mechanisms.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
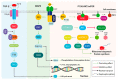
Figure 1
Parallel outline of several physiological roles of the TGFβ/p38, mitogen-activated protein kinase (MAPK), and P13k/AKT/mTOR signaling pathways. The p38 mitogen-activated kinase can be activated following upstream cytokine stimulation of the TGFβ pathway, which can subsequently activate TP53 in normal physiological conditions. TGFβ activation of p38 is not dependent on canonical SMAD signaling, but rather on the TAB/TAK1 complex and the MKK3/6 mitogen-activated protein kinase kinases. The canonical MAPK kinase pathway initiates with an extracellular stimulus in the form of growth factors (GFs) that bind and activate receptor tyrosine kinases (RTKs) on the cell membrane. Downstream activation of RAS, RAF and MEK in that order converge in the activation of the ERK1/2 transcription factor activator. The P13K/AKT/mTOR cascade can also be activated via RTKs and RAS, and its main implications are related to metabolic signaling and protein synthesis that sustain cell growth. TGFβ: transforming growth factor beta 1; p38: p38 kinase; P13k: phosphoinositide-3-kinase; AKT: v-akt murine thymoma viral oncogene homolog 1; mTOR: mechanistic target of rapamycin kinase; TAB: TGF-beta activated kinase 1 binding protein 2; TAK1: TGF-beta activated kinase 1; MKK3/6: mitogen-activated protein kinase kinase 3; RAS: small G-protein; RAF: Raf oncogene; MEK: MAP kinse-ERK kinase; RTKs: Receptor tyrosine kinases.
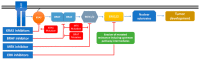
Figure 2
Mitogen-activated protein kinase (MAPK) inhibitor efficiency based on mutational status—cause of resistance and weak spots. Treatment resistance is a reoccurring problem in the case of MAPK pathway inhibitors. The post-treatment acquirement or selection of tumor cells with new mutations renders the treatment useless. In the case of KRAS, BRAF, and MEK inhibitors, mutations in any of these two components can determine therapeutic resistance and relapse. Targeting ERK can become a true Achilles heel in treating cancers with MAPK signaling alterations, as ERK inhibitors target specifically downstream of the signaling cascade, with no regard of the mutational status of the upstream components (e.g., KRAS and BRAF) (KRAS: Kirsten rat sarcoma viral oncogene homolog; BRAF: B-Raf proto-oncogene serine/threonine kinase; ERK:extracellular regulated MAP kinase).
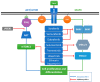
Figure 3
Targeted components of the MAPK and AKT signaling cascades by small-molecule inhibitors in cancer. Effective targeting of the pathway intermediates is an efficient tactic in the case of constitutively activated signaling cascades, such as the MAPK pathway in cancer. Successful inhibition of a step in the cascade impairs the downstream progression of the pathway and its overall aberrant function. Combinations of inhibitors or multi-targeting molecules are being investigated, as they might provide more efficient manipulation of the entire signaling pathway. (MAPK: mitogen-activated protein kinase AKT: v-akt murine thymoma viral oncogene homolog 1).
Similar articles
- Targeting Ras-ERK cascade by bioactive natural products for potential treatment of cancer: an updated overview.
Ali ES, Akter S, Ramproshad S, Mondal B, Riaz TA, Islam MT, Khan IN, Docea AO, Calina D, Sharifi-Rad J, Cho WC. Ali ES, et al. Cancer Cell Int. 2022 Aug 8;22(1):246. doi: 10.1186/s12935-022-02666-z. Cancer Cell Int. 2022. PMID: 35941592 Free PMC article. Review. - Extracellular-signal-regulated kinase/mitogen-activated protein kinase signaling as a target for cancer therapy: an updated review.
Najafi M, Ahmadi A, Mortezaee K. Najafi M, et al. Cell Biol Int. 2019 Nov;43(11):1206-1222. doi: 10.1002/cbin.11187. Epub 2019 Jul 11. Cell Biol Int. 2019. PMID: 31136035 Review. - Future perspectives in melanoma research: meeting report from the "Melanoma Bridge": Napoli, December 3rd-6th 2014.
Ascierto PA, Atkins M, Bifulco C, Botti G, Cochran A, Davies M, Demaria S, Dummer R, Ferrone S, Formenti S, Gajewski TF, Garbe C, Khleif S, Kiessling R, Lo R, Lorigan P, Arthur GM, Masucci G, Melero I, Mihm M, Palmieri G, Parmiani G, Puzanov I, Romero P, Schilling B, Seliger B, Stroncek D, Taube J, Tomei S, Zarour HM, Testori A, Wang E, Galon J, Ciliberto G, Mozzillo N, Marincola FM, Thurin M. Ascierto PA, et al. J Transl Med. 2015 Nov 30;13:374. doi: 10.1186/s12967-015-0736-1. J Transl Med. 2015. PMID: 26619946 Free PMC article. - The Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway as a Discovery Target in Stroke.
Sun J, Nan G. Sun J, et al. J Mol Neurosci. 2016 May;59(1):90-8. doi: 10.1007/s12031-016-0717-8. Epub 2016 Feb 3. J Mol Neurosci. 2016. PMID: 26842916 Review. - Advancements in MAPK signaling pathways and MAPK-targeted therapies for ameloblastoma: A review.
You Z, Liu SP, Du J, Wu YH, Zhang SZ. You Z, et al. J Oral Pathol Med. 2019 Mar;48(3):201-205. doi: 10.1111/jop.12807. Epub 2019 Feb 3. J Oral Pathol Med. 2019. PMID: 30489659 Review.
Cited by
- ARAF Amplification in Small-Cell Lung Cancer-Transformed Tumors Following Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors.
Kimura R, Adachi Y, Hirade K, Kisoda S, Yanase S, Shibata N, Ishii M, Fujiwara Y, Yamaguchi R, Fujita Y, Hosoda W, Ebi H. Kimura R, et al. Cancers (Basel). 2024 Oct 16;16(20):3501. doi: 10.3390/cancers16203501. Cancers (Basel). 2024. PMID: 39456595 Free PMC article. - Bioinformatic analysis reveals an exosomal miRNA-mRNA network in colorectal cancer.
Ma J, Wang P, Huang L, Qiao J, Li J. Ma J, et al. BMC Med Genomics. 2021 Feb 27;14(1):60. doi: 10.1186/s12920-021-00905-2. BMC Med Genomics. 2021. PMID: 33639954 Free PMC article. - The Histogenetic Origin of Malignant Cells Predicts Their Susceptibility towards Synthetic Lethality Utilizing the TK.007 System.
Pallasch FB, Freytag V, Kriegs M, Gatzemeier D, Mair T, Voss H, Riecken K, Dawood M, Fehse B, Efferth T, Schlüter H, Schumacher U. Pallasch FB, et al. Cancers (Basel). 2024 Jun 19;16(12):2278. doi: 10.3390/cancers16122278. Cancers (Basel). 2024. PMID: 38927982 Free PMC article. - Antitumor and Radiosensitizing Effects of Zinc Oxide-Caffeic Acid Nanoparticles against Solid Ehrlich Carcinoma in Female Mice.
Sayed HM, Said MM, Morcos NYS, El Gawish MA, Ismail AFM. Sayed HM, et al. Integr Cancer Ther. 2021 Jan-Dec;20:15347354211021920. doi: 10.1177/15347354211021920. Integr Cancer Ther. 2021. PMID: 34105411 Free PMC article. - Lactate induced up-regulation of KLHDC8A (Kelch domain-containing 8A) contributes to the proliferation, migration and apoptosis of human glioma cells.
Zhu X, Chen T, Yang H, Lv K. Zhu X, et al. J Cell Mol Med. 2020 Oct;24(20):11691-11702. doi: 10.1111/jcmm.15780. Epub 2020 Aug 26. J Cell Mol Med. 2020. PMID: 32851798 Free PMC article.
References
- Cancer Statistics. [(accessed on 15 October 2019)]; Available online: https://www.cancer.gov/about-cancer/understanding/statistics.
- Cainap C., Nagy V., Seicean A., Gherman A., Laszlo I., Lisencu C., Nadim A.H., Constantin A.M., Cainap S. Results of third-generation epirubicin/cisplatin/xeloda adjuvant chemotherapy in patients with radically resected gastric cancer. J. BU ON. 2016;21:349–359. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources