A Guide for the Use of the Ferret Model for Influenza Virus Infection - PubMed (original) (raw)
Review
A Guide for the Use of the Ferret Model for Influenza Virus Infection
Jessica A Belser et al. Am J Pathol. 2020 Jan.
Abstract
As influenza viruses continue to jump species barriers to cause human infection, assessments of disease severity and viral replication kinetics in vivo provide crucial information for public health professionals. The ferret model is a valuable resource for evaluating influenza virus pathogenicity; thus, understanding the most effective techniques for sample collection and usage, as well as the full spectrum of attainable data after experimental inoculation in this species, is paramount. This is especially true for scheduled necropsy of virus-infected ferrets, a standard component in evaluation of influenza virus pathogenicity, as necropsy findings can provide important information regarding disease severity and pathogenicity that is not otherwise available from the live animal. In this review, we describe the range of influenza viruses assessed in ferrets, the measures of experimental disease severity in this model, and optimal sample collection during necropsy of virus-infected ferrets. Collectively, this information is critical for assessing systemic involvement after influenza virus infection in mammals.
Published by Elsevier Inc.
Figures
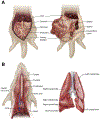
Figure 1
A: Abdominal organs in the ferret. Left panel: Visible organs on first entering the abdominal cavity include the liver, gastrointestinal (GI) tract, spleen, and urinary bladder. The pancreas lies closely adjacent to the pylorus and may not be readily visible without further handling of the GI tract. Right panel: With the GI tract moved to the side or lifted en bloc, the kidneys are found within the retroperitoneal space, along the dorsum, often embedded in adipose tissue. Differentiation of the intestinal segments may require removal of the GI tract en bloc, followed by separation of the loops for dissection and identification. The urinary bladder lies ventral to the rectum. B: Neck (cervical) and thoracic organs in the ferret. Left panel: Ventral midline incision through the soft tissues of the neck reveals the larynx, esophagus, and trachea (as well as thyroid gland and major vessels, not shown). The esophagus lies immediately dorsal to the trachea cranially and shifts toward the left as it courses caudally. The larynx is located at the cranial most end of the trachea. The caudal trachea bifurcates at the hilus of the lung, forming the mainstem bronchi as they enter the lungs. Removal of the ventral aspect of the ribcage reveals the lungs and heart. Right panel: The lungs in the ferret are composed of the cranial and caudal lobes on the left and cranial, middle, accessory, and caudal lobes on the right. Also shown are cut surfaces of the left lung lobes, revealing the spongy parenchyma and branching bronchi and vasculature. The tracheobronchial lymph nodes (not shown) are located at the hilus.
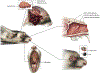
Figure 2
Tissues collected from the head include the conjunctiva and eye, soft palate, nasal turbinates, and brain. The method of brain removal shown is via a large opening along one side of the skull to allow lateral extraction of the entire brain. A sagittal section through the muzzle is shown to indicate the location of the nasal turbinates, which can be dislodged and removed using forceps via the nasal cavity. The conjunctiva is excised using a scalpel, and the eye is removed by proptosing the globe, detaching the associated connective tissue and muscle, and transecting the optic nerve. The soft palate is located deep in the back of the mouth and may require removal of the mandible for easier access.
Similar articles
- The ferret as a model organism to study influenza A virus infection.
Belser JA, Katz JM, Tumpey TM. Belser JA, et al. Dis Model Mech. 2011 Sep;4(5):575-9. doi: 10.1242/dmm.007823. Epub 2011 Aug 2. Dis Model Mech. 2011. PMID: 21810904 Free PMC article. Review. - Utility of Human In Vitro Data in Risk Assessments of Influenza A Virus Using the Ferret Model.
Creager HM, Kieran TJ, Zeng H, Sun X, Pulit-Penaloza JA, Holmes KE, Johnson AF, Tumpey TM, Maines TR, Beauchemin CAA, Belser JA. Creager HM, et al. J Virol. 2023 Jan 31;97(1):e0153622. doi: 10.1128/jvi.01536-22. Epub 2023 Jan 5. J Virol. 2023. PMID: 36602361 Free PMC article. - Exploring associations between viral titer measurements and disease outcomes in ferrets inoculated with 125 contemporary influenza A viruses.
Kieran TJ, Sun X, Maines TR, Beauchemin CAA, Belser JA. Kieran TJ, et al. J Virol. 2024 Feb 20;98(2):e0166123. doi: 10.1128/jvi.01661-23. Epub 2024 Jan 19. J Virol. 2024. PMID: 38240592 Free PMC article. - Detection of Airborne Influenza A and SARS-CoV-2 Virus Shedding following Ocular Inoculation of Ferrets.
Belser JA, Sun X, Kieran TJ, Brock N, Pulit-Penaloza JA, Pappas C, Basu Thakur P, Jones J, Wentworth DE, Zhou B, Tumpey TM, Maines TR. Belser JA, et al. J Virol. 2022 Dec 21;96(24):e0140322. doi: 10.1128/jvi.01403-22. Epub 2022 Nov 30. J Virol. 2022. PMID: 36448801 Free PMC article. - Complexities in Ferret Influenza Virus Pathogenesis and Transmission Models.
Belser JA, Eckert AM, Tumpey TM, Maines TR. Belser JA, et al. Microbiol Mol Biol Rev. 2016 Jul 13;80(3):733-44. doi: 10.1128/MMBR.00022-16. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27412880 Free PMC article. Review.
Cited by
- Interferon as an immunoadjuvant to enhance antibodies following influenza B infection and vaccination in ferrets.
Rowe T, Fletcher A, Svoboda P, Pohl J, Hatta Y, Jasso G, Wentworth DE, Ross TM. Rowe T, et al. NPJ Vaccines. 2024 Oct 24;9(1):199. doi: 10.1038/s41541-024-00973-2. NPJ Vaccines. 2024. PMID: 39448628 Free PMC article. - Pathogenic assessment of avian influenza viruses in migratory birds.
Kim EH, Kim YL, Kim SM, Yu KM, Casel MAB, Jang SG, Pascua PNQ, Webby RJ, Choi YK. Kim EH, et al. Emerg Microbes Infect. 2021 Dec;10(1):565-577. doi: 10.1080/22221751.2021.1899769. Emerg Microbes Infect. 2021. PMID: 33666526 Free PMC article. - Avian H7N9 influenza viruses are evolutionarily constrained by stochastic processes during replication and transmission in mammals.
Braun KM, Haddock Iii LA, Crooks CM, Barry GL, Lalli J, Neumann G, Watanabe T, Imai M, Yamayoshi S, Ito M, Moncla LH, Koelle K, Kawaoka Y, Friedrich TC. Braun KM, et al. Virus Evol. 2023 Jan 19;9(1):vead004. doi: 10.1093/ve/vead004. eCollection 2023. Virus Evol. 2023. PMID: 36814938 Free PMC article. - Influenza A virus polymerase acidic protein E23G/K substitutions weaken key baloxavir drug-binding contacts with minimal impact on replication and transmission.
Jones JC, Zagribelnyy B, Pascua PNQ, Bezrukov DS, Barman S, Okda F, Webby RJ, Ivanenkov YA, Govorkova EA. Jones JC, et al. PLoS Pathog. 2022 Jul 13;18(7):e1010698. doi: 10.1371/journal.ppat.1010698. eCollection 2022 Jul. PLoS Pathog. 2022. PMID: 35830486 Free PMC article. - Administration of antigenically distinct influenza viral particle combinations as an influenza vaccine strategy.
Zhu X, Luo Z, Leonard RA, Hamele CE, Spreng RL, Heaton NS. Zhu X, et al. PLoS Pathog. 2025 Jan 22;21(1):e1012878. doi: 10.1371/journal.ppat.1012878. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39841684 Free PMC article.
References
- Maher JA, DeStefano J: The ferret: an animal model to study influenza virus. Lab Anim 2004, 33:50–53 - PubMed
- Ng PS, Bohm R, Hartley-Tassell LE, Steen JA, Wang H, Lukowski SW, Hawthorne PL, Trezise AE, Coloe PJ, Grimmond SM, Haselhorst T, von Itzstein M, Paton AW, Paton JC, Jennings MP: Ferrets exclusively synthesize Neu5Ac and express naturally humanized influenza A virus receptors. Nat Commun 2014, 5:5750. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials