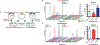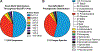Second messengers and divergent HD-GYP phosphodiesterases regulate 3',3'-cGAMP signaling - PubMed (original) (raw)
. 2020 Jan;113(1):222-236.
doi: 10.1111/mmi.14412. Epub 2019 Nov 17.
Affiliations
- PMID: 31665539
- PMCID: PMC7209772
- DOI: 10.1111/mmi.14412
Second messengers and divergent HD-GYP phosphodiesterases regulate 3',3'-cGAMP signaling
Todd A Wright et al. Mol Microbiol. 2020 Jan.
Abstract
3',3'-cyclic GMP-AMP (cGAMP) is the third cyclic dinucleotide (CDN) to be discovered in bacteria. No activators of cGAMP signaling have yet been identified, and the signaling pathways for cGAMP have been inferred to display a narrow distribution based upon the characterized synthases, DncV and Hypr GGDEFs. Here, we report that the ubiquitous second messenger cyclic AMP (cAMP) is an activator of the Hypr GGDEF enzyme GacB from Myxococcus xanthus. Furthermore, we show that GacB is inhibited directly by cyclic di-GMP, which provides evidence for cross-regulation between different CDN pathways. Finally, we reveal that the HD-GYP enzyme PmxA is a cGAMP-specific phosphodiesterase (GAP) that promotes resistance to osmotic stress in M. xanthus. A signature amino acid change in PmxA was found to reprogram substrate specificity and was applied to predict the presence of non-canonical HD-GYP phosphodiesterases in many bacterial species, including phyla previously not known to utilize cGAMP signaling.
© 2019 John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures
Figure 1.. Two distinct pathways for cGAMP signaling in bacteria.
Current cGAMP signaling pathways are found in deltaproteobacteria (red) and in the gammaproteobacterium Vibrio cholerae (blue). While the central second messenger, cGAMP, is identical, these pathways appear to use different classes of synthases, effectors, and phosphodiesterases. Text in red indicate new components revealed in this paper.
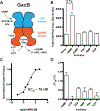
Figure 2.. GacB, a Hypr GGDEF enzyme, is activated by cAMP
(A) Schematic of GacB showing the allosteric site on the N-terminal GAF domain (blue) and predicted inhibitory (I-site) in the GGDEF domain (red). (B) Synthase activity of GacB (0.5 μM) with 1:1 ATP and GTP was measured by LC-MS in the absence or presence of candidate activators (1 μM). The sum of cGAMP and cdiG UV peak areas at 254 nm is reported, as the amount of cdiA produced was undetectable (p <0.05, n=2). (C) Synthase activity of GacB (5 nM) with GTP doped with trace [α−32P] GTP was measured by TLC in the presence of increasing amounts of cAMP (0–5 μM). Production of cdiG was quantified by 32P signal density and plotted versus [cAMP] (n=1). (D) Ligand binding to the GacB GAF domain was assessed by thermal shift assay using an MBP-GAF domain construct (1 μM). ΔTm was calculated as the difference between Tm with ligand (1 μM) and without ligand (n=3).
Figure 3.. GacB is selectively inhibited by cdiG
(A) Inhibition of GacB WT or I-site mutant (1 μM) by CDNs (10 μM) was measured by LC-MS (p<0.05, n=2). Due to potential in situ inhibition of GacB by other CDNs, cdiA production from ATP as sole substrate was used to quantify activity. The product cdiA (659 m/z) is reported as an ion-extracted signal from the mass spectra. For reactions testing cdiA as the candidate inhibitor, the background signal from added cdiA was subtracted (☨). (B) Determination of IC50 value for cdiG inhibition of GacB using LC-MS (n=3). (C) CDN binding to full-length GacB WT or I-site mutant was assessed by thermal shift assay using MBP-GacB (1 μM) and 1 and 10 μM of each CDN. ΔTm was calculated as the difference between Tm with CDN and without (n=3).
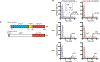
Figure 4.. Sequence and structural analysis of PmxA reveals signature active site variation
(A) Schematic of the PmxA (MXAN_2061) predicted homodimer. A single monomer contains an N-terminal sensory Cache domain, two TM helices, a coiled-coiled HAMP domain, and C-terminal HD-GYP domain with PDE activity. (B) Sequence alignment of PmxA with previously reported HD-GYP domain PDEs using the MUSCLE alignment tool. The canonical Rxx(R/K) motif is shown. PmxA has a Gln (Q) at the fourth position of the motif. (C) View of the active site of cdiG PDE PmGH (PDB 4MDZ) from Persephonella marina shows the two canonical motif residues, R314 and K317, forming hydrogen bond contacts with one of the two guanine nucleobases in cdiG. (D) Molecular diagrams that show the structurally characterized interaction of the PmGH RxxK motif with cdiG and the proposed interaction of PmxA RxxQ motif with cGAMP.
Figure 5.. PmxA is a selective cGAMP phosphodiesterase that can be reprogrammed via the signature residue
(A) Schematic of native PmxA and the MBP-tagged soluble PmxA construct used for in vitro activity assays, which was first reported by Skotnicka et al. (B) In vitro PDE activity of MBP-PmxA wt (blue) and Q518R mutant (red) was analyzed by LC-MS. Enzyme (10 μM) or control (no enzyme, black) was incubated with 50 μM of cGAMP (top), cdiG (middle), or cdiA (bottom) for 4 h at 30 °C (n=2). Representative UV traces for each reaction are shown, with CDN peaks labeled based on MS analysis. Inset highlights the observation of mononucleotide products for PmxA wt with cGAMP.
Figure 6.. PmxA has mild effects on type IV motility but promotes resistance to osmotic stress in M. xanthus
(A) Type IV pili (S) motility is mildly inhibited by cGAMP in M. xanthus, as shown by comparison of wild-type strain (DZ2) versus Δ_pmxA_ and Δ_gacB_ mutants. (B) The Δ_pmxA_ mutant forms fruiting bodies in high (+NaCl) or low (-NaCl) salt conditions, whereas fruiting body formation is inhibited in both wild-type (DZ2) and Δ_gacB_ strains under osmotic stress conditions. Interestingly, the Δ_pmxA_ strain forms fruiting bodies at the periphery of the swarm at low osmolarity and towards the center under high osmolarity.
Figure 7.. An RNA-based fluorescent biosensor assay for cyclic dinucleotide phosphodiesterases
(A) Schematic of RBF biosensor assay showing that a PDE candidate (yellow) and cGAMP (red) or cdiG (blue) biosensor-synthase plasmids are co-transformed and overexpressed in E. coli BL21* (DE3) cells. Active PDEs lead to a decrease in the bright cell population as analyzed by flow cytometry in the presence of the DFHBI dye. (B) Change in cellular cGAMP levels due to enzyme activity was measured by flow cytometry. Representative histograms show the dark (left) and bright (right) cell populations upon expression of WT PmxA (red), cdiG PDE PdeH (blue), and inactive PmxA mutant as a control (white). Y-axes are scaled by total cells (103), while the x-axes depict logarithmic fluorescence intensity. Bar graph depicts the change in % bright relative to the inactive control (p<0.05, n=3). (C) Change in cellular cdiG levels due to enzyme activity was measured by flow cytometry. Same as in part B (p<0.05, n=3), except the histogram scale for the dark cell population is 104.
Figure 8.. The variant signature Rxx(Q/N) motif is broadly distributed and most prevalent in Firmicutes and Proteobacteria
(A) The prevalence of the top 8 variants for the RxxX motif in bacterial HD-GYP domains is shown. The sequence analysis used 11,504 putative HD-GYP domains containing aligned RxxX motifs out of 14,326 total tBLASTn hits against the PmxA HD-GYP domain. (B) The distribution of RxxQ and RxxN HD-GYP motifs in Bacteria. A total of 539 sequences containing RxxQ or RxxN motifs were sorted based on unique species, leading to the identification of unique 215 bacterial species containing HD-GYP domains with these variant motifs. The species were categorized using NCBI Taxonomy.
Figure 9.. Identification of selective cGAMP phosphodiesterases (GAPs) from other bacteria, including one with the RxxN motif
(A) PDE activity screen of six candidate GAPs using the biosensor-based flow cytometry assay depicted in Fig. 7A. PDEs contain a Q (Mt: Moorella thermoacetica, Pp: Phaeobacter piscinae, Da: Dechloromonas aromatica, Gm: Geobacter metallireducens, Vt: Vibrio tapetis) or N (Bd: Bdellovibrio bacteriovorus) at the selectivity position. Activity of PDE candidates were compared to an inactive control, PmxA mutant (p < 0.05, n=3). (B) In vitro PDE activity of MBP-Bd2325 with different divalent metals was analyzed by LC-MS. Enzyme (10 μM) or control (no enzyme) was incubated with 50 μM of cGAMP or cdiG for 4 h at 30 °C in the presence of Mg2+, Mn2+, or Ni2+. A representative mass spectrum for each reaction is shown, with CDN or product peaks labeled based on MS analysis (n=2).
Similar articles
- Sequence Conservation, Domain Architectures, and Phylogenetic Distribution of the HD-GYP Type c-di-GMP Phosphodiesterases.
Galperin MY, Chou SH. Galperin MY, et al. J Bacteriol. 2022 Apr 19;204(4):e0056121. doi: 10.1128/jb.00561-21. Epub 2021 Dec 20. J Bacteriol. 2022. PMID: 34928179 Free PMC article. Review. - CRP-Like Transcriptional Regulator MrpC Curbs c-di-GMP and 3',3'-cGAMP Nucleotide Levels during Development in Myxococcus xanthus.
Kuzmich S, Blumenkamp P, Meier D, Szadkowski D, Goesmann A, Becker A, Søgaard-Andersen L. Kuzmich S, et al. mBio. 2021 Feb 22;13(1):e0004422. doi: 10.1128/mbio.00044-22. Epub 2022 Feb 15. mBio. 2021. PMID: 35164555 Free PMC article. - Structures of c-di-GMP/cGAMP degrading phosphodiesterase VcEAL: identification of a novel conformational switch and its implication.
Yadav M, Pal K, Sen U. Yadav M, et al. Biochem J. 2019 Nov 15;476(21):3333-3353. doi: 10.1042/BCJ20190399. Biochem J. 2019. PMID: 31647518 - DncV Synthesizes Cyclic GMP-AMP and Regulates Biofilm Formation and Motility in Escherichia coli ECOR31.
Li F, Cimdins A, Rohde M, Jänsch L, Kaever V, Nimtz M, Römling U. Li F, et al. mBio. 2019 Mar 5;10(2):e02492-18. doi: 10.1128/mBio.02492-18. mBio. 2019. PMID: 30837338 Free PMC article. - The ever-expanding world of bacterial cyclic oligonucleotide second messengers.
Yoon SH, Waters CM. Yoon SH, et al. Curr Opin Microbiol. 2021 Apr;60:96-103. doi: 10.1016/j.mib.2021.01.017. Epub 2021 Feb 25. Curr Opin Microbiol. 2021. PMID: 33640793 Free PMC article. Review.
Cited by
- RNA-based fluorescent biosensors for live cell imaging of small molecules and RNAs.
Su Y, Hammond MC. Su Y, et al. Curr Opin Biotechnol. 2020 Jun;63:157-166. doi: 10.1016/j.copbio.2020.01.001. Epub 2020 Feb 19. Curr Opin Biotechnol. 2020. PMID: 32086101 Free PMC article. Review. - The Signaling Pathway That cGAMP Riboswitches Found: Analysis and Application of Riboswitches to Study cGAMP Signaling in Geobacter sulfurreducens.
Tan Z, Chan CH, Maleska M, Banuelos Jara B, Lohman BK, Ricks NJ, Bond DR, Hammond MC. Tan Z, et al. Int J Mol Sci. 2022 Jan 21;23(3):1183. doi: 10.3390/ijms23031183. Int J Mol Sci. 2022. PMID: 35163114 Free PMC article. - Distinct dynamics and proximity networks of hub proteins at the prey-invading cell pole in a predatory bacterium.
Remy O, Santin YG, Jonckheere V, Tesseur C, Kaljević J, Van Damme P, Laloux G. Remy O, et al. J Bacteriol. 2024 Apr 18;206(4):e0001424. doi: 10.1128/jb.00014-24. Epub 2024 Mar 12. J Bacteriol. 2024. PMID: 38470120 Free PMC article. - Sequence Conservation, Domain Architectures, and Phylogenetic Distribution of the HD-GYP Type c-di-GMP Phosphodiesterases.
Galperin MY, Chou SH. Galperin MY, et al. J Bacteriol. 2022 Apr 19;204(4):e0056121. doi: 10.1128/jb.00561-21. Epub 2021 Dec 20. J Bacteriol. 2022. PMID: 34928179 Free PMC article. Review. - Fluorogenic RNA-Based Biosensors of Small Molecules: Current Developments, Uses, and Perspectives.
Kehrli J, Husser C, Ryckelynck M. Kehrli J, et al. Biosensors (Basel). 2024 Aug 1;14(8):376. doi: 10.3390/bios14080376. Biosensors (Basel). 2024. PMID: 39194605 Free PMC article. Review.
References
- Aravind L, Ponting CP (1997). The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends in Biochemical Sciences, 22(12), 458–459. - PubMed
- Burhenne H, Kaever V (2013). Quantification of Cyclic Dinucleotides by Reversed-Phase LC-MS/MS. Methods in Molecular Biology, 1016, 27–37. - PubMed
- Bustamante VH, Martínez-Flores I, Vlamakis HC, Zusman DR (2004). Analysis of the Frz signal transduction system of Myxococcus xanthus shows the importance of the conserved C-terminal region of the cytoplasmic chemoreceptor FrzCD in sensing signals. Molecular Microbiology, 53(5), 1501–1513. - PubMed
- Christen B, Christen M, Paul R, Schmid F, Folcher M, Jenoe P, et al. (2006). Allosteric control of cyclic di-GMP signaling. Journal of Biological Chemistry, 281(42), 32015–32024. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous