Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164 - PubMed (original) (raw)
Clinical Trial
. 2020 Jan 1;38(1):11-19.
doi: 10.1200/JCO.19.02107. Epub 2019 Nov 14.
Tae Won Kim 2, Eric Van Cutsem 3, Ravit Geva 4, Dirk Jäger 5, Hiroki Hara 6, Matthew Burge 7, Bert O'Neil 8, Petr Kavan 9, Takayuki Yoshino 10, Rosine Guimbaud 11, Hiroya Taniguchi 12, Elena Elez 13, Salah-Eddin Al-Batran 14, Patrick M Boland 15, Todd Crocenzi 16, Chloe E Atreya 17, Yi Cui 18, Tong Dai 19, Patricia Marinello 19, Luis A Diaz Jr 20, Thierry André 21
Affiliations
- PMID: 31725351
- PMCID: PMC7031958
- DOI: 10.1200/JCO.19.02107
Clinical Trial
Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164
Dung T Le et al. J Clin Oncol. 2020.
Abstract
Purpose: KEYNOTE-164 (NCT02460198) evaluated the antitumor activity of pembrolizumab in previously treated, metastatic, microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) colorectal cancer (CRC).
Methods: This phase II open-label study involved 128 centers worldwide. Eligible patients were age ≥ 18 years and had metastatic MSI-H/dMMR CRC treated with ≥ 2 prior lines of standard therapy, including fluoropyrimidine, oxaliplatin, and irinotecan with or without anti-vascular endothelial growth factor/epidermal growth factor receptor monoclonal antibody (cohort A) or ≥ 1 prior line of therapy (cohort B). MSI-H/dMMR status was assessed locally. Patients received pembrolizumab 200 mg every 3 weeks for up to 2 years until progression, unacceptable toxicity, or withdrawal. The primary end point was objective response rate by RECIST version 1.1 by independent central review. Secondary end points were duration of response, progression-free survival (PFS), overall survival, safety, and tolerability.
Results: A total of 124 patients with MSI-H/dMMR CRC (61 in cohort A, 63 in cohort B) enrolled. At data cutoff, median follow-up was 31.3 months (range, 0.2-35.6 months) for cohort A and 24.2 months (range, 0.1-27.1 months) for cohort B. Objective response rate was 33% (95% CI, 21% to 46%) and 33% (95% CI, 22% to 46%), respectively, with median duration of response not reached in either cohort. Median PFS was 2.3 months (95% CI, 2.1 to 8.1 months) and 4.1 months (95% CI, 2.1 to 18.9 months). Median overall survival was 31.4 months (95% CI, 21.4 months to not reached) and not reached (95% CI, 19.2 months to not reached). Treatment-related grade 3-4 adverse events occurred in 10 patients (16%) in cohort A and 8 (13%) in cohort B, with the most common occurring in ≥ 2 patients being pancreatitis, fatigue, increased alanine aminotransferase, and increased lipase (2 patients each; 3%) in cohort A.
Conclusion: Pembrolizumab is effective with a manageable safety profile in patients with MSI-H/dMMR CRC.
Figures
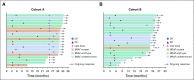
FIG 1.
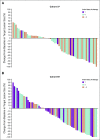
Best percent change from baseline in target lesion size (RECIST version 1.1 by central review) by prior lines of therapy in patients with microsatellite instability–high colorectal cancer in (A) cohort A and (B) cohort B. (*) Two patients in cohort A did not have postbaseline assessments and are not included. (†) One patient in cohort B did not have postbaseline assessments and is not included.
FIG 2.
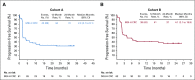
Kaplan-Meier estimates of progression-free survival (RECIST version 1.1 by central review) in patients with microsatellite instability–high colorectal cancer (MSI-H CRC) in (A) cohort A and (B) cohort B.
FIG 3.
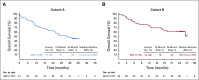
Kaplan-Meier estimates of overall survival in patients with microsatellite instability–high colorectal cancer (MSI-H CRC) in (A) cohort A and (B) cohort B. NR, not reached.
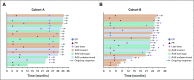
FIG A1.
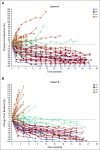
Percent change from baseline in target lesion size (RECIST v1.1) with time in patients with microsatellite instability–high/mismatch repair–deficient colorectal cancer in (A) Cohort A and (B) Cohort B. CR, complete response; PD, disease progression; PR, partial response; SD, stable disease.
FIG A2.
Treatment exposure and duration of response by BRAF status in patients with microsatellite instability–high/mismatch repair–deficient colorectal cancer in (A) Cohort A and (B) Cohort B. CR, complete response; PR, partial response.
FIG A3.
Treatment exposure and duration of response by RAS status in patients with microsatellite instability–high/mismatch repair–deficient colorectal cancer in (A) Cohort A and (B) Cohort B. CR, complete response; PR, partial response.
Comment in
- MSI-H: a truly agnostic biomarker?
Sidaway P. Sidaway P. Nat Rev Clin Oncol. 2020 Feb;17(2):68. doi: 10.1038/s41571-019-0310-5. Nat Rev Clin Oncol. 2020. PMID: 31831852 No abstract available.
Similar articles
- Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study.
Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Marabelle A, et al. J Clin Oncol. 2020 Jan 1;38(1):1-10. doi: 10.1200/JCO.19.02105. Epub 2019 Nov 4. J Clin Oncol. 2020. PMID: 31682550 Free PMC article. Clinical Trial. - A Phase II Study of Avelumab Monotherapy in Patients with Mismatch Repair-Deficient/Microsatellite Instability-High or POLE-Mutated Metastatic or Unresectable Colorectal Cancer.
Kim JH, Kim SY, Baek JY, Cha YJ, Ahn JB, Kim HS, Lee KW, Kim JW, Kim TY, Chang WJ, Park JO, Kim J, Kim JE, Hong YS, Kim YH, Kim TW. Kim JH, et al. Cancer Res Treat. 2020 Oct;52(4):1135-1144. doi: 10.4143/crt.2020.218. Epub 2020 Apr 24. Cancer Res Treat. 2020. PMID: 32340084 Free PMC article. Clinical Trial. - Pembrolizumab in Patients of Chinese Descent with Microsatellite Instability-high/Mismatch Repair Deficient Advanced Solid Tumors: KEYNOTE-158 Final Analysis.
Wu X, Mao Y, Xu N, Bai Y, Wang D, Chen X, Yin X, Deng Y, Yang J, Zhang J, Tang J, Huang Y, Li J, Luo S, Zheng H, Zhao W, Xu M, Li N, Mao Y, Gozman A, Xu J. Wu X, et al. Adv Ther. 2025 May;42(5):2480-2489. doi: 10.1007/s12325-025-03142-6. Epub 2025 Mar 22. Adv Ther. 2025. PMID: 40121391 Clinical Trial. - The EMA assessment of pembrolizumab as monotherapy for the first-line treatment of adult patients with metastatic microsatellite instability-high or mismatch repair deficient colorectal cancer.
Trullas A, Delgado J, Genazzani A, Mueller-Berghaus J, Migali C, Müller-Egert S, Zander H, Enzmann H, Pignatti F. Trullas A, et al. ESMO Open. 2021 Jun;6(3):100145. doi: 10.1016/j.esmoop.2021.100145. Epub 2021 Apr 30. ESMO Open. 2021. PMID: 33940347 Free PMC article. Review.
Cited by
- Neoadjuvant immunotherapy leads to complete pathologic response in locally advanced colon cancer.
Sandow L, Tsikitis L, Lopez CD, Brinkerhoff B, Kardosh A, Pegna G, Chen EY. Sandow L, et al. Clin Case Rep. 2024 Aug 6;12(8):e9218. doi: 10.1002/ccr3.9218. eCollection 2024 Aug. Clin Case Rep. 2024. PMID: 39114842 Free PMC article. - Immune Checkpoint Inhibition in Metastatic Colorectal Cancer Harboring Microsatellite Instability or Mismatch Repair Deficiency.
Cohen R, Colle R, Pudlarz T, Heran M, Duval A, Svrcek M, André T. Cohen R, et al. Cancers (Basel). 2021 Mar 8;13(5):1149. doi: 10.3390/cancers13051149. Cancers (Basel). 2021. PMID: 33800202 Free PMC article. Review. - Exploring Predictive and Prognostic Biomarkers in Colorectal Cancer: A Comprehensive Review.
Ashouri K, Wong A, Mittal P, Torres-Gonzalez L, Lo JH, Soni S, Algaze S, Khoukaz T, Zhang W, Yang Y, Millstein J, Lenz HJ, Battaglin F. Ashouri K, et al. Cancers (Basel). 2024 Aug 8;16(16):2796. doi: 10.3390/cancers16162796. Cancers (Basel). 2024. PMID: 39199569 Free PMC article. Review. - Peritoneal Recurrence of Cecal Cancer with Specific Imaging Findings and Shrinkage after Treatment with Pembrolizumab.
Takeda T, Shonaka T, Shimazaki R, Adachi Y, Otani M, Matsushita W, Tani C, Hasegawa K, Sumi Y. Takeda T, et al. J Anus Rectum Colon. 2022 Jan 28;6(1):67-71. doi: 10.23922/jarc.2021-053. eCollection 2022. J Anus Rectum Colon. 2022. PMID: 35128139 Free PMC article. - Clinical Validity of Plasma-Based Genotyping for Microsatellite Instability Assessment in Advanced GI Cancers: SCRUM-Japan GOZILA Substudy.
Nakamura Y, Okamoto W, Denda T, Nishina T, Komatsu Y, Yuki S, Yasui H, Esaki T, Sunakawa Y, Ueno M, Shinozaki E, Matsuhashi N, Ohta T, Kato K, Ohtsubo K, Bando H, Hara H, Satoh T, Yamazaki K, Yamamoto Y, Okano N, Terazawa T, Kato T, Oki E, Tsuji A, Horita Y, Hamamoto Y, Kawazoe A, Nakajima H, Nomura S, Mitani R, Yuasa M, Akagi K, Yoshino T. Nakamura Y, et al. JCO Precis Oncol. 2022 Feb;6:e2100383. doi: 10.1200/PO.21.00383. JCO Precis Oncol. 2022. PMID: 35188805 Free PMC article.
References
- International Agency for Research on Cancer: Cancer Fact Sheet, March 2019. https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sh....
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials