Body map proto-organization in newborn macaques - PubMed (original) (raw)
Body map proto-organization in newborn macaques
Michael J Arcaro et al. Proc Natl Acad Sci U S A. 2019.
Abstract
Topographic sensory maps are a prominent feature of the adult primate brain. Here, we asked whether topographic representations of the body are present at birth. Using functional MRI (fMRI), we find that the newborn somatomotor system, spanning frontoparietal cortex and subcortex, comprises multiple topographic representations of the body. The organization of these large-scale body maps was indistinguishable from those in older monkeys. Finer-scale differentiation of individual fingers increased over the first 2 y, suggesting that topographic representations are refined during early development. Last, we found that somatomotor representations were unchanged in 2 visually impaired monkeys who relied on touch for interacting with their environment, demonstrating that massive shifts in early sensory experience in an otherwise anatomically intact brain are insufficient for driving cross-modal plasticity. We propose that a topographic scaffolding is present at birth that both directs and constrains experience-driven modifications throughout somatosensory and motor systems.
Keywords: development; macaque; motor; proto-organization; somatosensory.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
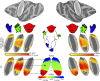
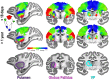
Fig. 1.
Topographic organization of primate central sulcus in young monkeys. Spatially specific activity within the central sulcus were observed during stimulation of the contralateral foot, hand, and face from both (middle row) GLM (beta values) and (bottom row) independent component (IC) analyses. Group average (n = 9 in right hemisphere and n = 7 in the left hemisphere) data show vertices that were significantly active in the beta (P < 0.0001, uncorrected) and IC (_z_ stat > 4.0) maps in at least 4 subjects from a conjunction analysis (
SI Appendix, Fig. S1
). (Bottom row, Middle) Combined, these activity maps formed an inverted topographic gradient of body representations symmetric between the hemispheres with the face (red) represented ventrally, feet (blue) represented dorsally, and a large hand representation (green) in between. Overlap between face and hand IC maps were represented as a gradient between red and green in RGB color space. Overlap between hand and foot IC maps were represented as a gradient between green and blue in RGB color space. The arrows indicate the correspondence between each body IC map to the topographic map. Monkey illustrations adapted from ref. , with permission from Elsevier.
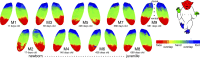
Fig. 2.
A large-scale topographic gradient of body representations spanning primary somatosensory and motor cortex was present in every monkey. A large-scale topographic gradient of body representations spanning primary somatosensory and motor cortex was present in every monkey. Maps are shown for each monkey arranged by age from (left) M1 at 11 d old to (right) M9 at 981 d old. See Fig. 1 for additional conventions.
Fig. 3.
Multiple body maps in newborns and juveniles. (A) Group average body maps of newborn (M1 and M2) and juvenile (M7 and M8) monkeys. Contralateral representations of the face, hand, and foot were found throughout frontal, anterior parietal, and insular cortex in monkeys younger than 18 d (M1 and M2) and in juveniles older than 1 y (M7 and M8). Several topographic gradients of body representations were observed in all monkeys. Data threshold at a group average z statistic > 4 for the strongest IC in each vertex. (B) Body representations overlapped with 23 areas of the Saleem and Logothetis atlas spanning motor, somatosensory, parietal, and insular cortex. (C) The spatial pattern of face, hand, and foot representations was consistent across all 9 monkeys tested. The dissimilarity matrix (1-corr) and MDS demonstrated that the spatial maps clustered based on body part stimulation, and there was no clear effect of age. For MDS, monkeys were luminance-coded by age from youngest (light) to oldest (dark). Face, hand, and foot representations were color coded red, green, and blue. See Fig. 1 for additional conventions.
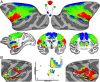
Fig. 4.
Distribution of body representations across cortex in newborns (M1 and M2) and juveniles (M7 and M8). The cortical area comprising (red) face, (green) hand, and (blue) foot representations was comparable between newborns and juveniles for 23 cortical areas identified from the Saleem and Logothetis atlas (79) shown in Fig. 3 A and B. Large, black-outlined circles denote average body part representations (percentage of area) based on the grouping of the 23 cortical areas overlapping our somatomotor maps into broader somatosensory, motor, parietal, and insular cortical regions as shown in Fig. 3. Smaller circles denote average age group body part representations (percentage of area) within individual cortical areas.
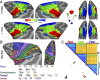
Fig. 5.
Subcortical body map organization in newborns and juveniles. Representations of the contralateral body were found in the putamen, globus pallidus, and ventral posterior nucleus of the thalamus in newborns (M1 and M2), and these maps were comparable to the organization in juvenile monkeys (M7 and M8). Data threshold at a group average z statistic > 4 for the strongest IC in each vertex. See Fig. 1 for additional conventions.
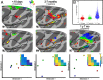
Fig. 6.
Digit representations in newborns and juveniles. (A) With the exception of the thumb and index fingers, monkeys younger than 18 d old (M1 and M2) lacked spatially distinct representations of contralateral fingers in primary somatosensory cortex. In monkeys 2–7 mo of age (M3 and M4), representations of each digit were identified in primary (3a/b) and secondary (SII) somatosensory cortex. At 1 y 7 mo of age (M7), digit representations appeared more robust in primary and secondary somatosensory cortex and were also found within motor cortex. The black outline illustrates the extent of the group average hand IC map. MDS was computed across all voxels within the group average hand representation in the central sulcus. Data threshold of P < 0.0001, uncorrected, for the digit representation with the largest beta value. (B) Distribution of evoked responses from digit stimulation in the hand representation of primary somato-motor cortex for newborns, 2–7-mo-old, and 1-y 7-mo-old monkeys. Red line illustrates median, black line illustrates mean.
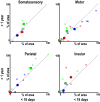
Fig. 7.
No clear cross-modal reorganization in monkeys raised under visual form deprivation. (A) Body maps were found in 2 monkeys (M5 and M6) raised without visual form experience for the first year of life across (Top) cortex and (Bottom) subcortex. These monkeys primarily rely on touch for navigation and interacting with their environment. Despite this massive shift in sensory experience relative to control monkeys, the body map organization was indistinguishable from controls. (B) Digit representations were found in these monkeys comparable to juvenile control monkeys (Fig. 6 and
SI Appendix, Fig. S7
). Somatomotor activity was not observed in occipital cortex, suggesting that such drastic changes in early sensory experience were not sufficient to cause large-scale cross-modal rewiring. Body mapping threshold at z stat > 4.0 and digit mapping data threshold of P < 0.0001, uncorrected. See Fig. 1 for additional conventions.
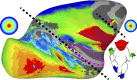
Fig. 8.
Importance of being topographic. Topographic representations of sensory spaces cover most of the cortical surface. As illustrated by a group average (n = 6) eccentricity map (32), the posterior half of the brain and an anterior part of the arcuate sulcus (FEF) comprise retinotopic representations of visual space. As illustrated by a group average (n = 8) body map, anterior parietal, frontal, and insular cortex comprise topographic representations of the body. These retinotopic (62) and somatomotor (current study) maps are present at birth. The black dashed lines differentiate retinotopic and somatomotor body maps. As illustrated by the magenta dashed circle, several tonotopic maps span the lower bank of the lateral sulcus (81).
Similar articles
- Differential effects of the mode of touch, active and passive, on experience-driven plasticity in the S1 cutaneous digit representation of adult macaque monkeys.
Cybulska-Klosowicz A, Tremblay F, Jiang W, Bourgeon S, Meftah EM, Chapman CE. Cybulska-Klosowicz A, et al. J Neurophysiol. 2020 Mar 1;123(3):1072-1089. doi: 10.1152/jn.00014.2019. Epub 2020 Feb 5. J Neurophysiol. 2020. PMID: 32023143 - Whole brain mapping of somatosensory responses in awake marmosets investigated with ultra-high-field fMRI.
Cléry JC, Hori Y, Schaeffer DJ, Gati JS, Pruszynski JA, Everling S. Cléry JC, et al. J Neurophysiol. 2020 Dec 1;124(6):1900-1913. doi: 10.1152/jn.00480.2020. Epub 2020 Oct 28. J Neurophysiol. 2020. PMID: 33112698 - Mapping human somatosensory cortex in individual subjects with 7T functional MRI.
Sanchez-Panchuelo RM, Francis S, Bowtell R, Schluppeck D. Sanchez-Panchuelo RM, et al. J Neurophysiol. 2010 May;103(5):2544-56. doi: 10.1152/jn.01017.2009. Epub 2010 Feb 17. J Neurophysiol. 2010. PMID: 20164393 Free PMC article. - Somatosensory maps.
Harding-Forrester S, Feldman DE. Harding-Forrester S, et al. Handb Clin Neurol. 2018;151:73-102. doi: 10.1016/B978-0-444-63622-5.00004-8. Handb Clin Neurol. 2018. PMID: 29519481 Review. - Some anatomical bases of cortical somatotopic organization.
Woolsey TA. Woolsey TA. Brain Behav Evol. 1978;15(5-6):325-71. doi: 10.1159/000123786. Brain Behav Evol. 1978. PMID: 737526 Review. No abstract available.
Cited by
- Against cortical reorganisation.
Makin TR, Krakauer JW. Makin TR, et al. Elife. 2023 Nov 21;12:e84716. doi: 10.7554/eLife.84716. Elife. 2023. PMID: 37986628 Free PMC article. - Functional Reorganization After Four-Week Brain-Computer Interface-Controlled Supernumerary Robotic Finger Training: A Pilot Study of Longitudinal Resting-State fMRI.
Liu Y, Huang S, Wang Z, Ji F, Ming D. Liu Y, et al. Front Neurosci. 2022 Feb 11;15:766648. doi: 10.3389/fnins.2021.766648. eCollection 2021. Front Neurosci. 2022. PMID: 35221886 Free PMC article. - Brainwide mesoscale functional networks revealed by focal infrared neural stimulation of the amygdala.
Ping A, Wang J, García-Cabezas MÁ, Li L, Zhang J, Gothard KM, Zhu J, Roe AW. Ping A, et al. bioRxiv [Preprint]. 2024 Jun 6:2024.02.14.580397. doi: 10.1101/2024.02.14.580397. bioRxiv. 2024. PMID: 38464165 Free PMC article. Preprint. - A comprehensive macaque fMRI pipeline and hierarchical atlas.
Jung B, Taylor PA, Seidlitz J, Sponheim C, Perkins P, Ungerleider LG, Glen D, Messinger A. Jung B, et al. Neuroimage. 2021 Jul 15;235:117997. doi: 10.1016/j.neuroimage.2021.117997. Epub 2021 Mar 28. Neuroimage. 2021. PMID: 33789138 Free PMC article. - Robotic hand augmentation drives changes in neural body representation.
Kieliba P, Clode D, Maimon-Mor RO, Makin TR. Kieliba P, et al. Sci Robot. 2021 May 19;6(54):eabd7935. doi: 10.1126/scirobotics.abd7935. Sci Robot. 2021. PMID: 34043536 Free PMC article.
References
- Kaas J. H., Topographic maps are fundamental to sensory processing. Brain Res. Bull. 44, 107–112 (1997). - PubMed
- Raos V., Franchi G., Gallese V., Fogassi L., Somatotopic organization of the lateral part of area F2 (dorsal premotor cortex) of the macaque monkey. J. Neurophysiol. 89, 1503–1518 (2003). - PubMed
- Strick P. L., Preston J. B., Multiple representation in the primate motor cortex. Brain Res. 154, 366–370 (1978). - PubMed
- Wong Y. C., Kwan H. C., MacKay W. A., Murphy J. T., Spatial organization of precentral cortex in awake primates. I. Somatosensory inputs. J. Neurophysiol. 41, 1107–1119 (1978). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources