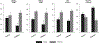Renal Effects of Sodium-Glucose Co-Transporter Inhibitors - PubMed (original) (raw)
Review
Renal Effects of Sodium-Glucose Co-Transporter Inhibitors
Scott C Thomson et al. Am J Cardiol. 2019.
Abstract
Sodium-glucose co-transporter 2 (SGLT2) inhibitors immediately reduce the glomerular filtration rate (GFR) in patients with type 2 diabetes mellitus. When given chronically, they confer benefit by markedly slowing the rate at which chronic kidney disease progresses and are the first agents to do so since the advent of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs). Salutary effects on the kidney were first demonstrated in cardiovascular outcomes trials and have now emerged from trials enriched in subjects with type 2 diabetes mellitus and chronic kidney disease. A simple model that unifies the immediate and long-term effects of SGLT2 inhibitors on kidney function is based on the assumption that diabetic hyperfiltration puts the kidney at long-term risk and evidence that hyperfiltration is an immediate response to a reduced signal for tubuloglomerular feedback, which occurs to the extent that SGLT2 activity mediates a primary increase in sodium and fluid reabsorption by the proximal tubule. This model will likely continue to serve as a useful description accounting for the beneficial effect of SGLT2 inhibitors on the diabetic kidney, similar to the hemodynamic explanation for the benefit of ACEIs and ARBs. A more complex model will be required to incorporate positive interactions between SGLT2 and sodium-hydrogen exchanger 3 in the proximal tubule and between sodium-glucose co-transporter 1 (SGLT1) and nitric oxide synthase in the macula densa. The implication of these latter nuances for day-to-day clinical medicine remains to be determined.
Keywords: Diabetic kidney disease; Glomerular filtration; Proximal tubule; Tubuloglomerular feedback.
Copyright © 2019. Published by Elsevier Inc.
Conflict of interest statement
Conflicts of Interest: SCT has received research support from Merck and Pfizer. VV has served as a consultant and received honoraria from AstraZeneca, Boehringer Ingelheim, Intarcia Therapeutics, Janssen, Eli Lilly, and Merck and received grant support for investigator-initiated research from Boehringer Ingelheim, Astra-Zeneca, Fresenius, Janssen, and Bayer.
Figures
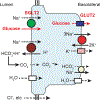
Figure 1
SGLT2 mechanism in early proximal tubule. SGLT2, bicarbonate reabsorption, and sodium-potassium ATPase, which provides sodium potential to drive both, are shown. GLUT2 = glucose transporter 2; SGLT2 = sodium-glucose co-transporter 2.
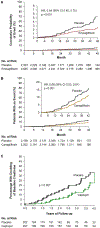
Figure 2
Renal outcomes in SGLT2 inhibitor trials of (A) empagliflozin (EMPA-REG OUTCOME) and (B) canagliflozin (CREDENCE) compared with (C) an earlier RAAS blocker (captopril) trial. (A) Renal-specific composite outcome of doubling of SCr level, initiation of renal replacement therapy, or death from renal disease in the EMPA-REG OUTCOME trial. From New England Journal of Medicine, Wanner C, et al., Empagliflozin and progression of kidney disease in type 2 diabetes, 375, 323–334. Copyright © 2016 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. (B) Renal-specific composite outcome of ESRD, doubling of SCr level, or renal death in the CREDENCE trial. From New England Journal of Medicine, Perkovic V, et al. for the CREDENCE Trial Investigators, Canagliflozin and renal outcomes in type 2 diabetes and nephropathy, 380, 2295–2306. Copyright © 2019 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. The placebo group was already receiving an ACEI or ARB and their rate of poor outcomes is remarkably similar to those who received captopril in the 1993 trial (~15% at 3.5 years). (C) Renal end point of doubling of SCr level to ≥2.0 mg/dL in the 1993 captopril trial. From New England Journal of Medicine, Lewis EJ, et al., The effect of angiotensin-convertingenzyme inhibition on diabetic nephropathy, 329, 1456–1462, Copyright © 1993 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; RAAS = renin-angiotensin-aldosterone system; SCr = serum creatinine; SGLT2 = sodium-glucose co-transporter 2.
Figure 3
Similarity of eGFR outcomes in SGLT2 inhibitor clinical trials of (A) empagliflozin (EMPA-REG OUTCOME) and (B) canagliflozin (CREDENCE) compared with (C) an earlier RAAS blocker (enalapril) trial. Patients destined to benefit from SGLT2 inhibitor or ACEI treatment show an immediate decline in eGFR, which remains stable thereafter, whereas eGFR continues to decline in the comparator group. (A) Adjusted means for the eGFR over a period of 192 weeks in the EMPA-REG OUTCOME trial. From New England Journal of Medicine, Wanner C, et al., Empagliflozin and progression of kidney disease in type 2 diabetes, 375, 323–334. Copyright © 2016 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. (B) Change from the screening level in eGFR in the on-treatment population in the CREDENCE trial. The I bars indicate the standard error. From New England Journal of Medicine, Perkovic V, et al. for the CREDENCE Trial Investigators, Canagliflozin and renal outcomes in type 2 diabetes and nephropathy, 380, 2295–2306. Copyright © 2019 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. (C) Time course of eGFR before, during, and after withdrawal of antihypertensive treatment: in group A (•), patients who initially showed a distinct fall in GFR, and in group B (°), patients in whom eGFR did not fall after start of treatment in the enalapril trial. This figure shows a rebound increase in eGFR on cessation of enalapril treatment; this rebound also occurs with cessation of treatment with SGLT2 inhibitors (data not shown). Reprinted from Kidney International, 51 (3), Apperloo AJ, et al., A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function, 793–797, Copyright 1997, with permission from Elsevier and International Society of Nephrology. ACEI = angiotensin-converting enzyme inhibitor; eGFR = estimated glomerular filtration rate; Empa = empagliflozin; RAAS = renin-angiotensin-aldosterone system; SGLT2 = sodium-glucose co-transporter 2.
Figure 4
Immediate effects of phlorizin on macula densa delivery of Na, Cl, fluid volume, and SNGFR measured downstream of the macula densa to allow TGF to operate. Phlorizin was delivered to Bowman’s space. Cl = chloride; MD = macula densa; Na = sodium; SNGFRd = single-nephron glomerular filtration rate, determined by distal tubular collection; TGF = tubuloglomerular feedback; VED = early distal tubular flow rate. Data from Vallon V et al. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–76.
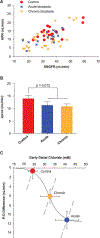
Figure 5
Acute effects and chronic effects with SGLT2 blockade on tubular reabsorption in Wistar Froemter rats with early diabetes. (A) Individual late proximal tubular fluid collections showing APR as a function of SNGFR. (B) Proximal reabsorption adjusted for SNGFR by ANCOVA (APR). Collections were made without perfusing Henle’s loop, so each point represents the shoulder of a TGF curve. (C) Degree of TGF activation (P-D difference [proximal minus distal]) as a function of the TGF stimulus (CED). The solid curve is a hyperbolic tangent parameterized to represent the acute TGF response. The dashed lines represent the forward effects of SNGFR on CED as a result of glomerulotubular balance. Superimposed on this graph are the operating points (mean § SE) for the 3 phases of SGLT2 blockade in Wistar Froemter rats. This figure illustrates 2 salient features of the transition from acute to chronic SGLT2 blockade. ANCOVA = analysis of covariance; APR = absolute proximal reabsorption; CED = early distal chloride concentration; SE = standard error; SGLT2 = sodium-glucose co-transporter 2; SNGFR = single-nephron glomerular filtration rate; TGF = tubuloglomerular feedback. Adapted from Thomson SC, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012; 302: R75–R83. Copyright © 2012, The American Physiological Society.
Similar articles
- The tubular hypothesis of nephron filtration and diabetic kidney disease.
Vallon V, Thomson SC. Vallon V, et al. Nat Rev Nephrol. 2020 Jun;16(6):317-336. doi: 10.1038/s41581-020-0256-y. Epub 2020 Mar 9. Nat Rev Nephrol. 2020. PMID: 32152499 Free PMC article. Review. - Changes in Proximal Tubular Reabsorption Modulate Microvascular Regulation via the TGF System.
Poursharif S, Hamza S, Braam B. Poursharif S, et al. Int J Mol Sci. 2022 Sep 23;23(19):11203. doi: 10.3390/ijms231911203. Int J Mol Sci. 2022. PMID: 36232506 Free PMC article. Review. - Knockout of Na+-glucose cotransporter SGLT1 mitigates diabetes-induced upregulation of nitric oxide synthase NOS1 in the macula densa and glomerular hyperfiltration.
Song P, Huang W, Onishi A, Patel R, Kim YC, van Ginkel C, Fu Y, Freeman B, Koepsell H, Thomson S, Liu R, Vallon V. Song P, et al. Am J Physiol Renal Physiol. 2019 Jul 1;317(1):F207-F217. doi: 10.1152/ajprenal.00120.2019. Epub 2019 May 15. Am J Physiol Renal Physiol. 2019. PMID: 31091127 Free PMC article. - Evaluation of renal and cardiovascular protection mechanisms of SGLT2 inhibitors: model-based analysis of clinical data.
Hallow KM, Greasley PJ, Helmlinger G, Chu L, Heerspink HJ, Boulton DW. Hallow KM, et al. Am J Physiol Renal Physiol. 2018 Nov 1;315(5):F1295-F1306. doi: 10.1152/ajprenal.00202.2018. Epub 2018 Jul 18. Am J Physiol Renal Physiol. 2018. PMID: 30019930 Free PMC article. - SGLT2 Inhibitors and the Diabetic Kidney.
Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. Fioretto P, et al. Diabetes Care. 2016 Aug;39 Suppl 2:S165-71. doi: 10.2337/dcS15-3006. Diabetes Care. 2016. PMID: 27440829
Cited by
- Modern Challenges in Type 2 Diabetes: Balancing New Medications with Multifactorial Care.
Caturano A, Galiero R, Rocco M, Tagliaferri G, Piacevole A, Nilo D, Di Lorenzo G, Sardu C, Vetrano E, Monda M, Marfella R, Rinaldi L, Sasso FC. Caturano A, et al. Biomedicines. 2024 Sep 7;12(9):2039. doi: 10.3390/biomedicines12092039. Biomedicines. 2024. PMID: 39335551 Free PMC article. Review. - Taboo in cardiology: renin-angiotensin-aldosterone system antagonists worsening renal failure.
Cice G, Calo' L. Cice G, et al. Eur Heart J Suppl. 2024 Apr 17;26(Suppl 1):i49-i52. doi: 10.1093/eurheartjsupp/suae017. eCollection 2024 Apr. Eur Heart J Suppl. 2024. PMID: 38867878 Free PMC article. - Renal handling of albumin in rats with early stage diabetes: A theoretical analysis.
Edwards A. Edwards A. J Physiol. 2024 Jul;602(14):3575-3592. doi: 10.1113/JP286245. Epub 2024 Jun 10. J Physiol. 2024. PMID: 38857419 - SGLT2-independent effects of canagliflozin on NHE3 and mitochondrial complex I activity inhibit proximal tubule fluid transport and albumin uptake.
Albalawy WN, Youm EB, Shipman KE, Trull KJ, Baty CJ, Long KR, Rbaibi Y, Wang XP, Fagunloye OG, White KA, Jurczak MJ, Kashlan OB, Weisz OA. Albalawy WN, et al. Am J Physiol Renal Physiol. 2024 Jun 1;326(6):F1041-F1053. doi: 10.1152/ajprenal.00005.2024. Epub 2024 Apr 25. Am J Physiol Renal Physiol. 2024. PMID: 38660713 - Evaluation and post-transplant management of children after multi-organ-with-kidney transplantation.
Engen RM, Lemoine CP. Engen RM, et al. Pediatr Nephrol. 2024 Oct;39(10):2875-2885. doi: 10.1007/s00467-024-06336-2. Epub 2024 Mar 14. Pediatr Nephrol. 2024. PMID: 38483593 Review.
References
- Stiles P, Lusk G. On the action of phlorizin. Am J Phys 1903;10:61–79.
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733–94. - PubMed
- Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care 2015;38:355–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK106102/DK/NIDDK NIH HHS/United States
- R01 HL119836/HL/NHLBI NIH HHS/United States
- R01 DK112042/DK/NIDDK NIH HHS/United States
- P30 DK079337/DK/NIDDK NIH HHS/United States
- R01 HL142814/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical