Accuracy in detecting inadequate research reporting by early career peer reviewers using an online CONSORT-based peer-review tool (COBPeer) versus the usual peer-review process: a cross-sectional diagnostic study - PubMed (original) (raw)
doi: 10.1186/s12916-019-1436-0.
Anthony Chauvin 1 2 3, David Moher 6, David Schriger 7, Sally Hopewell 8, Daniel Shanahan 9, Sabina Alam 10, Gabriel Baron 4 5, Jean-Philippe Regnaux 4 5, Perrine Crequit 4 5, Valeria Martinez 11, Carolina Riveros 4 5, Laurence Le Cleach 12, Alessandro Recchioni 13, Douglas G Altman 8, Isabelle Boutron 4 5
Affiliations
- PMID: 31744489
- PMCID: PMC6864983
- DOI: 10.1186/s12916-019-1436-0
Accuracy in detecting inadequate research reporting by early career peer reviewers using an online CONSORT-based peer-review tool (COBPeer) versus the usual peer-review process: a cross-sectional diagnostic study
Anthony Chauvin et al. BMC Med. 2019.
Abstract
Background: The peer review process has been questioned as it may fail to allow the publication of high-quality articles. This study aimed to evaluate the accuracy in identifying inadequate reporting in RCT reports by early career researchers (ECRs) using an online CONSORT-based peer-review tool (COBPeer) versus the usual peer-review process.
Methods: We performed a cross-sectional diagnostic study of 119 manuscripts, from BMC series medical journals, BMJ, BMJ Open, and Annals of Emergency Medicine reporting the results of two-arm parallel-group RCTs. One hundred and nineteen ECRs who had never reviewed an RCT manuscript were recruited from December 2017 to January 2018. Each ECR assessed one manuscript. To assess accuracy in identifying inadequate reporting, we used two tests: (1) ECRs assessing a manuscript using the COBPeer tool (after completing an online training module) and (2) the usual peer-review process. The reference standard was the assessment of the manuscript by two systematic reviewers. Inadequate reporting was defined as incomplete reporting or a switch in primary outcome and considered nine domains: the eight most important CONSORT domains and a switch in primary outcome(s). The primary outcome was the mean number of domains accurately classified (scale from 0 to 9).
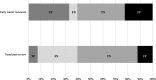
Results: The mean (SD) number of domains (0 to 9) accurately classified per manuscript was 6.39 (1.49) for ECRs using COBPeer versus 5.03 (1.84) for the journal's usual peer-review process, with a mean difference [95% CI] of 1.36 [0.88-1.84] (p < 0.001). Concerning secondary outcomes, the sensitivity of ECRs using COBPeer versus the usual peer-review process in detecting incompletely reported CONSORT items was 86% [95% CI 82-89] versus 20% [16-24] and in identifying a switch in primary outcome 61% [44-77] versus 11% [3-26]. The specificity of ECRs using COBPeer versus the usual process to detect incompletely reported CONSORT domains was 61% [57-65] versus 77% [74-81] and to identify a switch in primary outcome 77% [67-86] versus 98% [92-100].
Conclusions: Trained ECRs using the COBPeer tool were more likely to detect inadequate reporting in RCTs than the usual peer review processes used by journals. Implementing a two-step peer-review process could help improve the quality of reporting.
Trial registration: Clinical.Trials.gov NCT03119376 (Registered April, 18, 2017).
Keywords: CONSORT statement; Peer reviewers; Randomized controlled trials; Reporting.
Conflict of interest statement
The authors have completed the ICMJE uniform disclosure form. Author AR is the Senior Editor of BMC Medicine and thus recused himself from the handling of this article at this journal. All other authors declare that they have no competing interests.
Figures
Fig. 1
Example of the CONSORT-based peer-review tool (COBPeer)
Fig. 2
Example of the CONSORT-based peer-review tool (COBPeer)
Fig. 3
Proportions of items evaluated by early career reviewers and usual peer review classified as true positive (TP), false negative (FN), true negative (TN), and false positive (FP)
Similar articles
- A protocol of a cross-sectional study evaluating an online tool for early career peer reviewers assessing reports of randomised controlled trials.
Chauvin A, Moher D, Altman D, Schriger DL, Alam S, Hopewell S, Shanahan DR, Recchioni A, Ravaud P, Boutron I. Chauvin A, et al. BMJ Open. 2017 Sep 15;7(9):e017462. doi: 10.1136/bmjopen-2017-017462. BMJ Open. 2017. PMID: 28918414 Free PMC article. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Reminding Peer Reviewers of Reporting Guideline Items to Improve Completeness in Published Articles: Primary Results of 2 Randomized Trials.
Speich B, Mann E, Schönenberger CM, Mellor K, Griessbach AN, Dhiman P, Gandhi P, Lohner S, Agarwal A, Odutayo A, Puebla I, Clark A, Chan AW, Schlussel MM, Ravaud P, Moher D, Briel M, Boutron I, Schroter S, Hopewell S. Speich B, et al. JAMA Netw Open. 2023 Jun 1;6(6):e2317651. doi: 10.1001/jamanetworkopen.2023.17651. JAMA Netw Open. 2023. PMID: 37294569 Free PMC article. - Are peer reviewers encouraged to use reporting guidelines? A survey of 116 health research journals.
Hirst A, Altman DG. Hirst A, et al. PLoS One. 2012;7(4):e35621. doi: 10.1371/journal.pone.0035621. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558178 Free PMC article. - Relation of completeness of reporting of health research to journals' endorsement of reporting guidelines: systematic review.
Stevens A, Shamseer L, Weinstein E, Yazdi F, Turner L, Thielman J, Altman DG, Hirst A, Hoey J, Palepu A, Schulz KF, Moher D. Stevens A, et al. BMJ. 2014 Jun 25;348:g3804. doi: 10.1136/bmj.g3804. BMJ. 2014. PMID: 24965222 Free PMC article. Review.
Cited by
- Transparency and reporting characteristics of COVID-19 randomized controlled trials.
Kapp P, Esmail L, Ghosn L, Ravaud P, Boutron I. Kapp P, et al. BMC Med. 2022 Sep 26;20(1):363. doi: 10.1186/s12916-022-02567-y. BMC Med. 2022. PMID: 36154932 Free PMC article. - SPIRIT 2025 statement: Updated guideline for protocols of randomised trials.
Chan AW, Boutron I, Hopewell S, Moher D, Schulz KF, Collins GS, Tunn R, Aggarwal R, Berkwits M, Berlin JA, Bhandari N, Butcher NJ, Campbell MK, Chidebe RCW, Elbourne DR, Farmer AJ, Fergusson DA, Golub RM, Goodman SN, Hoffmann TC, Ioannidis JPA, Kahan BC, Knowles RL, Lamb SE, Lewis S, Loder E, Offringa M, Ravaud P, Richards DP, Rockhold FW, Schriger DL, Siegfried NL, Staniszewska S, Taylor RS, Thabane L, Torgerson DJ, Vohra S, White IR, Hróbjartsson A. Chan AW, et al. PLoS Med. 2025 Apr 28;22(4):e1004589. doi: 10.1371/journal.pmed.1004589. eCollection 2025 Apr. PLoS Med. 2025. PMID: 40294521 Free PMC article. - The PRISMA 2020 statement: An updated guideline for reporting systematic reviews.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. Page MJ, et al. PLoS Med. 2021 Mar 29;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. eCollection 2021 Mar. PLoS Med. 2021. PMID: 33780438 Free PMC article. - Assessment of transparency and selective reporting of interventional trials studying colorectal cancer.
Pellat A, Boutron I, Ravaud P. Pellat A, et al. BMC Cancer. 2022 Mar 15;22(1):278. doi: 10.1186/s12885-022-09334-5. BMC Cancer. 2022. PMID: 35291962 Free PMC article. - Development of a checklist to detect errors in meta-analyses in systematic reviews of interventions: study protocol.
Kanukula R, Page M, Dwan K, Turner S, Loder E, Mayo-Wilson E, Li T, Misra A, McDonald S, Forbes A, McKenzie J. Kanukula R, et al. F1000Res. 2021 Jun 8;10:455. doi: 10.12688/f1000research.53034.1. eCollection 2021. F1000Res. 2021. PMID: 34249342 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous