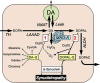
3,4-Dihydroxyphenylacetaldehyde Is More Efficient than Dopamine in Oligomerizing and Quinonizing α-Synuclein - PubMed (original) (raw)
3,4-Dihydroxyphenylacetaldehyde Is More Efficient than Dopamine in Oligomerizing and Quinonizing _α_-Synuclein
Yunden Jinsmaa et al. J Pharmacol Exp Ther. 2020 Feb.
Abstract
Lewy body diseases such as Parkinson's disease involve intraneuronal deposition of the protein _α_-synuclein (AS) and depletion of nigrostriatal dopamine (DA). Interactions of AS with DA oxidation products may link these neurohistopathologic and neurochemical abnormalities via two potential pathways: spontaneous oxidation of DA to dopamine-quinone and enzymatic oxidation of DA catalyzed by monoamine oxidase to form 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is then oxidized to DOPAL-Q. We compared these two pathways in terms of the ability of DA and DOPAL to modify AS. DOPAL was far more potent than DA both in oligomerizing and forming quinone-protein adducts with (quinonizing) AS. The DOPAL-induced protein modifications were enhanced similarly by pro-oxidation with Cu(II) or tyrosinase and inhibited similarly by antioxidation with _N_-acetylcysteine. Dopamine oxidation evoked by Cu(II) or tyrosinase did not quinonize AS. In cultured MO3.13 human oligodendrocytes DOPAL resulted in the formation of numerous intracellular quinoproteins that were visualized by near-infrared spectroscopy. We conclude that of the two routes by which oxidation of DA modifies AS and other proteins the route via DOPAL is more prominent. The results support developing experimental therapeutic strategies that might mitigate deleterious modifications of proteins such as AS in Lewy body diseases by targeting DOPAL formation and oxidation. SIGNIFICANCE STATEMENT: Interactions of the protein _α_-synuclein with products of dopamine oxidation in the neuronal cytoplasm may link two hallmark abnormalities of Parkinson disease: Lewy bodies (which contain abundant AS) and nigrostriatal DA depletion (which produces the characteristic movement disorder). Of the two potential routes by which DA oxidation may alter AS and other proteins, the route via the autotoxic catecholaldehyde 3,4-dihydroxyphenylacetaldehyde is more prominent; the results support experimental therapeutic strategies targeting DOPAL formation and DOPAL-induced protein modifications.
U.S. Government work not protected by U.S. copyright.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Graphical abstract
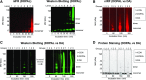
Fig. 1.
Time courses of DOPAL and DA effects on AS quinonization and oligomerization. (A) AS (3 _μ_M) was incubated with DOPAL or DA (30 _μ_M each) at 37°C and samples were taken after 120 minutes (A) and 300 minutes (B) of incubation. (A and B) Quinonized AS was detected by nIRF spectroscopy (red). (A and C) Oligomerized AS was detected by western blotting (green). (D) Protein staining was used to demonstrate decreased AS monomer during incubation of AS with DOPAL. Lanes = order of the gel lanes, Groups: 1 = AS alone as a control (CON), 2 = DOPAL, 3 = DA. DOPAL time dependently increased AS quinonization and oligomerization, whereas DA did not elicit AS quinonization and produced a slight smear of high molecular weight AS.
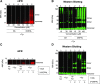
Fig. 2.
Effects of enzymatic oxidation of DA and DOPAL with tyrosinase on AS quinonization and oligomerization. DA or DOPAL (30 _μ_M each) was incubated with tyrosinase (+Tyr) or without tyrosinase (no Tyr) for 20 minutes at room temperature and then incubated with AS (3 _μ_M) for 1 hour at 37°C. (A and B) Concentration course of DA or DOPAL (10, 30, and 100 _μ_M each) oxidation with Tyr. (A and C) Quinonized AS was detected by nIRF spectroscopy (red). (B and D) Oligomerized AS was detected by western blotting (green). N = number of replicates; 1 = DA; 2 = DOPAL. Enzymatic oxidization augmented DOPAL-induced oligomerization and quinonization of AS. Incubation of AS with DA and Tyr resulted in a smear of high molecular weight AS. DA did not quinonize AS even in the setting of enzymatic oxidation by Tyr.
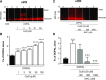
Fig. 3.
Effects of Cu(II) on DOPAL-induced AS quinonization and NAC effect. (A and B) AS (3 _μ_M) was incubated with 30 _μ_M DOPAL and 1–100 _μ_M Cu(II) for 1 hour at 37°C. (C and D) AS was incubated with DOPAL and 30 _μ_M Cu(II) and 0–1000 μ_M NAC for 1 hour at 37°C. Quinonized AS was detected by nIRF spectroscopy (red). N, number of replicates; Fx, fractions of integrated intensities of AS monomers compared with DOPAL alone. Statistical analyses were done by one-way ANOVA with Dunnett’s post-hoc test. Mean values are expressed as ± S.E.M. ****P < 0.0001, ***P < 0.001 compared with DOPAL alone; ☨☨☨_P < 0.001 vs. DOPAL + Cu(II) compared with no NAC. Cu(II) concentration dependently augmented DOPAL-induced AS quinonization and oligomerization. NAC attenuated this effect.
Fig. 4.
Comparisons of DA- vs. DOPAL-induced AS modifications in the presence of Cu(II). (A) Incubation of AS (3 _µ_M) with 30 _µ_M each of DA and DOPAL and 1 _µ_M Cu(II). (B) Incubation of AS with DA or DOPAL and 30 _µ_M Cu(II). (A–C) Quinonized AS was detected by nIRF spectroscopy (red). (A and B) Oligomerized AS was detected by western blotting (green). ΔIntegrated intensity, the difference in integrated intensity of signal at each time point minus the integrated intensity at 0 minutes; Lanes, order of the gel lanes. Cu(II) at 30 _µ_M accelerated and enhanced DOPAL-induced oligomerization and quinonization of AS. Incubation of Cu(II) (30 _µ_M) with 30 _µ_M DA and AS resulted in a smear of high molecular weight AS.
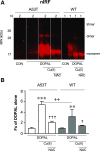
Fig. 5.
(A and B) DOPAL-induced quinonization of mutant A53T vs. WT AS. WT or A53T mutant AS (3 _μ_M) was incubated with 30 μ_M each of DOPAL and Cu(II) (with or without) or 300 μ_M NAC (with or without) for 1 hour at 37°C. Quinonized AS was detected by nIRF spectroscopy. Fx, fractions of integrated intensities of AS monomers compared with DOPAL alone; N, number of replicates. Statistical analyses were done by one-way ANOVA with Dunnett’s post-hoc test. Mean values are expressed as ± S.E.M. ***P < 0.001; **P < 0.01 compared with DOPAL alone; ☨☨☨_P < 0.001; ☨☨_P < 0.05 compared with no NAC; ++P < 0.01 for A53T compared with WT. DOPAL quinonized both A53T and WT AS, with about twice as large an effect on A53T AS. The enhancing effects were attenuated by NAC.
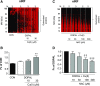
Fig. 6.
DOPAL-induced quinonization of intracellular proteins in MO3.13 cells and NAC effect. (A and B) MO3.13 cells (1.5 × 105 cells/well) were exposed to DOPAL (100 _μ_M) or DOPAL + Cu(II) (10 and 30 _μ_M) for 24 hours and then lysed in radioimmunoprecipitation assay buffer with protease inhibitors. (C and D) MO3.13 cells were exposed to DOPAL and 30 µ_M Cu(II), with NAC (0–300 μ_M) added at the start of incubation. DOPAL-quinonized proteins were detected and quantified by nIRF spectroscopy (red). Fx, fractions of integrated intensities of each column compared with CON (B) or DOPAL alone (D) groups normalized to the protein of each lanes. N, number of replicates. Statistical analyses were done by one-way ANOVA with Dunnett’s post-hoc test. Mean values are expressed as ± S.E.M. **P < 0.001 compared with DOPAL alone; ☨☨☨_P < 0.001; ☨☨_P < 0.01 compared with no NAC. Cu(II) augmented DOPAL-induced quinonization of intracellular proteins and NAC attenuated these effects.
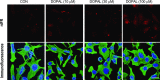
Fig. 7.
Visualization of intracellular DOPAL-induced quinoproteins. MO3.13 cells were cultured in slide chambers (8 × 104 cells/slides) for 24 hours and treated with Cu(II) (30 _μ_M) and 0–100 _μ_M DOPAL for 5 hours. Cells were then stained with 4,6-diamidino-2-phenylindole (1:2000) (blue) and human tubulin antibody (1:1500) (green). Immunofluorescence and nIRF were visualized microscopically. Scale bar in images is 20 _μ_m. Treatment with DOPAL produced nIRF signals, suggesting the presence of quinoproteins.
Similar articles
- Divalent metal ions enhance DOPAL-induced oligomerization of alpha-synuclein.
Jinsmaa Y, Sullivan P, Gross D, Cooney A, Sharabi Y, Goldstein DS. Jinsmaa Y, et al. Neurosci Lett. 2014 May 21;569:27-32. doi: 10.1016/j.neulet.2014.03.016. Epub 2014 Mar 23. Neurosci Lett. 2014. PMID: 24670480 Free PMC article. - The Catecholaldehyde Hypothesis for the Pathogenesis of Catecholaminergic Neurodegeneration: What We Know and What We Do Not Know.
Goldstein DS. Goldstein DS. Int J Mol Sci. 2021 Jun 1;22(11):5999. doi: 10.3390/ijms22115999. Int J Mol Sci. 2021. PMID: 34206133 Free PMC article. Review. - Oligomerization and Membrane-binding Properties of Covalent Adducts Formed by the Interaction of α-Synuclein with the Toxic Dopamine Metabolite 3,4-Dihydroxyphenylacetaldehyde (DOPAL).
Follmer C, Coelho-Cerqueira E, Yatabe-Franco DY, Araujo GD, Pinheiro AS, Domont GB, Eliezer D. Follmer C, et al. J Biol Chem. 2015 Nov 13;290(46):27660-79. doi: 10.1074/jbc.M115.686584. Epub 2015 Sep 17. J Biol Chem. 2015. PMID: 26381411 Free PMC article. - Oxidative Transformations of 3,4-Dihydroxyphenylacetaldehyde Generate Potential Reactive Intermediates as Causative Agents for Its Neurotoxicity.
Ito S, Tanaka H, Ojika M, Wakamatsu K, Sugumaran M. Ito S, et al. Int J Mol Sci. 2021 Oct 29;22(21):11751. doi: 10.3390/ijms222111751. Int J Mol Sci. 2021. PMID: 34769179 Free PMC article. - The catecholaldehyde hypothesis: where MAO fits in.
Goldstein DS. Goldstein DS. J Neural Transm (Vienna). 2020 Feb;127(2):169-177. doi: 10.1007/s00702-019-02106-9. Epub 2019 Dec 5. J Neural Transm (Vienna). 2020. PMID: 31807952 Free PMC article. Review.
Cited by
- Stable expression of the human dopamine transporter in N27 cells as an in vitro model for dopamine cell trafficking and metabolism.
Cagle BS, Sturgeon ML, O'Brien JB, Wilkinson JC, Cornell RA, Roman DL, Doorn JA. Cagle BS, et al. Toxicol In Vitro. 2021 Oct;76:105210. doi: 10.1016/j.tiv.2021.105210. Epub 2021 Jul 5. Toxicol In Vitro. 2021. PMID: 34252731 Free PMC article. - Disease-modifying treatment of Parkinson's disease by phytochemicals: targeting multiple pathogenic factors.
Naoi M, Maruyama W, Shamoto-Nagai M. Naoi M, et al. J Neural Transm (Vienna). 2022 Jun;129(5-6):737-753. doi: 10.1007/s00702-021-02427-8. Epub 2021 Oct 15. J Neural Transm (Vienna). 2022. PMID: 34654977 Review. - Cannabidiol Displays Proteomic Similarities to Antipsychotics in Cuprizone-Exposed Human Oligodendrocytic Cell Line MO3.13.
Falvella ACB, Smith BJ, Silva-Costa LC, Valença AGF, Crunfli F, Zuardi AW, Hallak JE, Crippa JA, de Almeida V, Martins-de-Souza D. Falvella ACB, et al. Front Mol Neurosci. 2021 May 28;14:673144. doi: 10.3389/fnmol.2021.673144. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34122009 Free PMC article. - Rasagiline and selegiline modulate mitochondrial homeostasis, intervene apoptosis system and mitigate α-synuclein cytotoxicity in disease-modifying therapy for Parkinson's disease.
Naoi M, Maruyama W, Shamoto-Nagai M. Naoi M, et al. J Neural Transm (Vienna). 2020 Feb;127(2):131-147. doi: 10.1007/s00702-020-02150-w. Epub 2020 Jan 28. J Neural Transm (Vienna). 2020. PMID: 31993732 Review. - Renalase Overexpression-Mediated Excessive Metabolism of Peripheral Dopamine, DOPAL Accumulation, and α-Synuclein Aggregation in Baroreflex Afferents Contribute to Neuronal Degeneration and Autonomic Dysfunction.
Xiong X, Xu YZ, Zhang Y, Zhang HF, Dou TM, Li XY, Xu ZY, Cui CP, Li XL, Li BY. Xiong X, et al. Biomedicines. 2025 May 20;13(5):1243. doi: 10.3390/biomedicines13051243. Biomedicines. 2025. PMID: 40427069 Free PMC article.
References
- Asanuma M, Miyazaki I, Ogawa N. (2003) Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res 5:165–176. - PubMed
- Badillo-Ramírez I, Saniger JM, Rivas-Arancibia S. (2019) 5-S-cysteinyl-dopamine, a neurotoxic endogenous metabolite of dopamine: implications for Parkinson’s disease. Neurochem Int 129:104514. - PubMed
- Banerjee K, Munshi S, Sen O, Pramanik V, Roy Mukherjee T, Chakrabarti S. (2014) Dopamine cytotoxicity involves both oxidative and nonoxidative pathways in SH-SY5Y cells: potential role of alpha-synuclein overexpression and proteasomal inhibition in the etiopathogenesis of Parkinson’s disease. Parkinsons Dis 2014:878935. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials