Engineering Nanoparticles to Reprogram the Tumor Immune Microenvironment for Improved Cancer Immunotherapy - PubMed (original) (raw)
Review
. 2019 Oct 17;9(26):7981-8000.
doi: 10.7150/thno.37568. eCollection 2019.
Affiliations
- PMID: 31754376
- PMCID: PMC6857062
- DOI: 10.7150/thno.37568
Review
Engineering Nanoparticles to Reprogram the Tumor Immune Microenvironment for Improved Cancer Immunotherapy
Madiha Saeed et al. Theranostics. 2019.
Abstract
Immunotherapy is rapidly maturing towards extensive clinical use. However, it does not work well in large patient populations because of an immunosuppressed microenvironment and limited reinvigoration of antitumor immunity. The tumor microenvironment is a complex milieu in which the principles of physiology and anatomy are defied and which is considered an immune-privileged site promoting T cell exhaustion. Tremendous research interest exists in developing nanoparticle-based approaches to modulate antitumor immune responses. The increasing use of immunotherapies in the clinic requires robust programming of immune cells to boost antitumor immunity. This review summarizes recent advances in the engineering of nanoparticles for improved anticancer immunotherapy. It discusses emerging nanoparticle-based approaches for the modulation of tumor cells and immune cells, such as dendritic cells, T cells and tumor-associated macrophages, with the intention to overcome challenges currently faced in the clinic. Furthermore, this review describes potentially curative combination therapeutic approaches to provoke effective tumor antigen-specific immune responses. We foresee a future in which improvement in patient's surveillance will become a mainstream practice.
Keywords: Cancer immunotherapy; antitumor immune response.; immune microenvironment programming; nanoparticles.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Figure 1
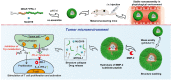
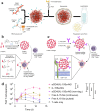
Schematic illustration of nanoparticle-mediated modulation of immune response to improve the effectiveness of cancer immunotherapy. Engineered nanoparticles are taken up and escape the endosome to release a variety of cargoes (such as antigens and adjuvants) in tumor immune microenvironment and lymph nodes whereas the negative regulatory pathways are deactivated by immune-checkpoint inhibitors.
Figure 2
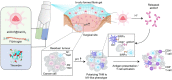
Composition and the proposed antitumor mechanism of the NLG919@DEAP-DPPA-1 nanoparticle. DEAP-DPPA-1 and NLG919 were co-assembled into a nanoparticle and maintained stable nanostructure at the physiological environment. In acidic tumor conditions, the hydrophobic core of the nanoparticle allowed MMP-2 to hydrolyze its substrate peptide leading to the complete dissociation of the nanostructure. Thereafter, NLG919 and MDPPA-1 were released for blocking the immunosuppressive pathways, IDO and PD-L1, respectively. Adapted with permission from , copyright 2018 American Chemical Society.
Figure 3
Schematic illustration of the BCPN for improved immunotherapy. (a) Self-assembly procedure of BCPN nanoparticles. (b) Schematic illustration of BCPN capable of modulating the immune response by eliciting antitumor immunity while suppressing regulatory T cells. Adapted with permission from , copyright 2018 John Wiley and Sons.
Figure 4
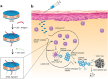
Schematic showing the in situ sprayed bioresponsive fibrin gel containing anti-CD47@CaCO3 NPs within the post-surgery tumor bed. Anti-CD47@CaCO3 encapsulated in fibrin scavenged H+ in the surgical wound site and precisely released anti-CD47 and thereby promoted the polarization of TAMs (M1-like phenotype) as well as the blockade of the 'don't eat me' signal in cancer cells. Adapted with permission from , copyright 2019 Nature Publishing Group.
Figure 5
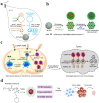
Design of high-density lipoprotein (sHDL) nanodisc platform for personalized cancer vaccines. (a) sHDL nanodiscs, composed of apolipoprotein-1 mimetic peptides (22A) and phospholipids, were engineered to co-deliver antigen (Ag) peptides and adjuvants. The sHDL nanodiscs were mixed with cysteine-modified Ag peptides, including tumor neoantigens that were identified through DNA sequencing of tumor exome and then incubated with a cholesterol-modified immunostimulatory oligonucleotide (Cho-CpG) and subsequent formation of Ag and CpG co-loaded sHDL nanodiscs (sHDL-Ag/CpG). (b) Following administration, sHDL nanodiscs ensured effective co-delivery of Ag and CpG to draining lymph nodes, promoted potent and durable Ag presentation by DCs (Signal 1), and induced DCs maturation (Signal 2), which resulted in stimulation of robust Ag-specific CD8 α+ cytotoxic T-lymphocyte (CTL) responses. Activated CTLs can recognize and kill the targeted tumor cells in peripheral tissues as well as exert potent antitumor responses. Combination immunotherapy with immune checkpoint blockade, i.e., anti-PD-1 and anti-CTLA further improved the efficacy of nanodisc vaccination, leading to the eradication of established tumors. Adapted with permission from , copyright 2016 Nature Publishing Group.
Figure 6
(a) Schematic of iDR-NC/neoantigen nanovaccines for synergistic tumor immunotherapy. The concurrent rolling circle replication (RCR) and rolling circle transcription (RCT) in the same solution producing tandem CpG and STAT3 shRNA, which were self-assembled into intertwining DNA-RNA MFs. (b) The above MFs were shrunk due to the presence of PPT-g-PEG and formed iDR-NCs, which was further loaded with tumor-specific neoantigen via hydrophobic interactions between hydrophobic PPT moieties and peptide antigens. (c) In immunocompetent mice, iDR-NCs/neoantigen complexes were delivered into APCs in draining LNs, which ultimately inhibited tumor growth by eliciting the strong and durable neoantigen-specific T cell responses. Adapted with permission from , copyright 2017 Nature Publishing Group (d) Schematic of the minimalist design of the PC7A nanovaccine. PC7A nanovaccine enhanced the antigen delivery, cross-presentation, and activated the STING pathway to robust T cell activation and to boost antitumor immunity for cancer immunotherapy. Adapted with permission from , copyright 2017 Nature Publishing Group.
Figure 7
(a) Schematic of the T cell-targeted DNA nanocarrier. The fabrication of NPs is demonstrated. The two plasmids encoding for 194-1BBz CAR and the iPB7 transposase were encapsulated into the nanoparticles. Adapted with permission from , copyright 2017 Nature Publishing Group. (b) Schematic of the synthesis of protein NG and protein release in response to reducing conditions of local microenvironment. (c) Scheme for surface modification of cytokine-NGs that ensured effective and stable anchoring on T cell surfaces. (d) Fold expansion of naive CD8+ T cells that were stimulated with anti-CD3/CD28 beads in the presence of surface-bound aCD45/IL-15Sa-NGs, IL-15Sa-NGs or nondegradable NGs (aCD45/IL-15SaNGs(non-deg.)) or that were incubated with free IL-15Sa. Adapted with permission from , copyright 2018 Nature Publishing Group.
Figure 8
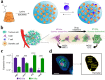
(a) Schematic of CDNP preparation by lysine crosslinking of succinyl- β-cyclodextrin (orange) and subsequent drug loading by guest-host complexation of R848 (blue). (b) Schematic overview of the tumor microenvironment, where TAMs were mainly canonically M2-like; however, their behavior was pharmacologically influenced. (c) Gene expression of M2-like (IL-4 treated) and M1-like (LPS/INF-γ treated) murine macrophages. (d) Raw images were processed by automated segmentation for the measurement of prominent features useful in the identification of M1-like polarization as indicated in yellow, where the mean radius (solid line), minor axis length (dotted line) and perimeter (dashed line). Cells were stained for nuclei (DAPI, red), actin (phalloidin, green), and cell membrane (WGA, blue). Scale bar, 25 μm. Adapted with permission from , copyright 2018 Nature Publishing Group.
Figure 9
Schematic Illustration of the acid-activatable micelleplexes for PD-L1 blockade-enhanced photodynamic cancer immunotherapy (a) Chemical structure of the acid-activatable POP micelleplexes that were co-loaded with PPa and siRNA; the micelleplexes dissociated at an acidic microenvironment that were attributed to the protonation of the of PDPA. (b) Schematic of POP-PD-L1 micelleplex mediated combined cancer immunotherapy. Upon PDT, the POP-PD-L1 micelleplexes generated ROS, which ultimately induced an adaptive immune response by provoking HSP70 and NF-κB pathways, triggered the secretion of the pro-inflammatory cytokine, and recruited tumor-infiltrating T cells. The antitumor immune response was further improved by RNAi of PD-L1. Adapted with permission from , 2016 American Chemical Society.
Figure 10
Schematic illustration of the prodrug vesicles for improved cancer immunotherapy by combing ICD induction and CD47 blockade. (a) Schematic design of the dual-responsive, e.g., acidity and MMP-2 prodrug vesicles. (b) Simplified mechanism of NP-mediated chemo-immunotherapy and CD47 blockade, which inhibited tumor growth, distant metastasis, and relapse. Adapted with permission from , copyright 2019 John Wiley and Sons.
Similar articles
- Nano-Immune-Engineering Approaches to Advance Cancer Immunotherapy: Lessons from Ultra-pH-Sensitive Nanoparticles.
Li S, Bennett ZT, Sumer BD, Gao J. Li S, et al. Acc Chem Res. 2020 Nov 17;53(11):2546-2557. doi: 10.1021/acs.accounts.0c00475. Epub 2020 Oct 16. Acc Chem Res. 2020. PMID: 33063517 Free PMC article. - Immunomodulatory nanoparticles activate cytotoxic T cells for enhancement of the effect of cancer immunotherapy.
Wells K, Liu T, Zhu L, Yang L. Wells K, et al. Nanoscale. 2024 Oct 3;16(38):17699-17722. doi: 10.1039/d4nr01780c. Nanoscale. 2024. PMID: 39257225 Review. - Engineered Nanomaterials for Tumor Immune Microenvironment Modulation in Cancer Immunotherapy.
Xing H, Li X. Xing H, et al. Chemistry. 2024 Jun 6;30(32):e202400425. doi: 10.1002/chem.202400425. Epub 2024 May 2. Chemistry. 2024. PMID: 38576219 Review. - Non-viral gene delivery for cancer immunotherapy.
Wang W, Saeed M, Zhou Y, Yang L, Wang D, Yu H. Wang W, et al. J Gene Med. 2019 Jul;21(7):e3092. doi: 10.1002/jgm.3092. Epub 2019 May 28. J Gene Med. 2019. PMID: 30991453 Review. - Regulation of cancer-immunity cycle and tumor microenvironment by nanobiomaterials to enhance tumor immunotherapy.
Yang J, Zhang C. Yang J, et al. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020 Jul;12(4):e1612. doi: 10.1002/wnan.1612. Epub 2020 Mar 1. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020. PMID: 32114718 Review.
Cited by
- Biomedical nanomaterials for immunological applications: ongoing research and clinical trials.
Lenders V, Koutsoumpou X, Sargsian A, Manshian BB. Lenders V, et al. Nanoscale Adv. 2020 Aug 24;2(11):5046-5089. doi: 10.1039/d0na00478b. eCollection 2020 Nov 11. Nanoscale Adv. 2020. PMID: 36132021 Free PMC article. Review. - Nanoparticle-Based Chimeric Antigen Receptor Therapy for Cancer Immunotherapy.
Shin S, Lee P, Han J, Kim SN, Lim J, Park DH, Paik T, Min J, Park CG, Park W. Shin S, et al. Tissue Eng Regen Med. 2023 Jun;20(3):371-387. doi: 10.1007/s13770-022-00515-8. Epub 2023 Mar 3. Tissue Eng Regen Med. 2023. PMID: 36867402 Free PMC article. Review. - Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies.
Liu L, Kshirsagar PG, Gautam SK, Gulati M, Wafa EI, Christiansen JC, White BM, Mallapragada SK, Wannemuehler MJ, Kumar S, Solheim JC, Batra SK, Salem AK, Narasimhan B, Jain M. Liu L, et al. Theranostics. 2022 Jan 1;12(3):1030-1060. doi: 10.7150/thno.64805. eCollection 2022. Theranostics. 2022. PMID: 35154473 Free PMC article. Review. - Nanotheranostics in Breast Cancer Bone Metastasis: Advanced Research Progress and Future Perspectives.
Miao L, Zhu Y, Chang H, Zhang X. Miao L, et al. Pharmaceutics. 2024 Nov 21;16(12):1491. doi: 10.3390/pharmaceutics16121491. Pharmaceutics. 2024. PMID: 39771471 Free PMC article. Review. - Modulating barriers of tumor microenvironment through nanocarrier systems for improved cancer immunotherapy: a review of current status and future perspective.
Lan H, Zhang W, Jin K, Liu Y, Wang Z. Lan H, et al. Drug Deliv. 2020 Dec;27(1):1248-1262. doi: 10.1080/10717544.2020.1809559. Drug Deliv. 2020. PMID: 32865029 Free PMC article. Review.
References
- Wang H, Mooney DJ. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat Mater. 2018;17:761–772. - PubMed
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources