Earth's earliest and deepest purported fossils may be iron-mineralized chemical gardens - PubMed (original) (raw)
Earth's earliest and deepest purported fossils may be iron-mineralized chemical gardens
Sean McMahon. Proc Biol Sci. 2019.
Abstract
Recognizing fossil microorganisms is essential to the study of life's origin and evolution and to the ongoing search for life on Mars. Purported fossil microbes in ancient rocks include common assemblages of iron-mineral filaments and tubes. Recently, such assemblages have been interpreted to represent Earth's oldest body fossils, Earth's oldest fossil fungi, and Earth's best analogues for fossils that might form in the basaltic Martian subsurface. Many of these putative fossils exhibit hollow circular cross-sections, lifelike (non-crystallographic, constant-thickness, and bifurcate) branching, anastomosis, nestedness within 'sheaths', and other features interpreted as strong evidence for a biological origin, since no abiotic process consistent with the composition of the filaments has been shown to produce these specific lifelike features either in nature or in the laboratory. Here, I show experimentally that abiotic chemical gardening can mimic such purported fossils in both morphology and composition. In particular, chemical gardens meet morphological criteria previously proposed to establish biogenicity, while also producing the precursors to the iron minerals most commonly constitutive of filaments in the rock record. Chemical gardening is likely to occur in nature. Such microstructures should therefore not be assumed to represent fossil microbes without independent corroborating evidence.
Keywords: astrobiology; biomorphs; chemical gardens; fossil bacteria; fossil fungi.
Conflict of interest statement
The author declares no competing interests.
Figures
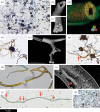
Figure 1.
Photomicrographs and scanning electron micrographs of experimental iron-mineralizing chemical gardens. (a) Numerous straight and irregularly curved siliceous filaments attached to the knob-like remnants of iron sulfate seed grains less than 63 µm in diameter. (b) Siliceous filament showing rough iron (oxyhydr)oxide-coated interior with hollow central cavity. (c) Siliceous filament with the central cavity filled by iron (oxyhydr)oxides. (d) Siliceous filament with laminated wall; overlaid energy-dispersive X-ray (EDX) spectroscopy data show iron-rich innermost layers (red/yellow). (e) Multiple siliceous branching filaments radiating from a seed grain remnant; the brown colour is contributed by ferric iron. (f) Branching siliceous tube with minimal inner coating. (g) Siliceous filaments with variable yellow/brown inner coating showing branch (arrowed). (h) Anastomosing siliceous filaments; arrows indicate direction of growth. (i) Broken siliceous filament (arrowed) on the interior of a larger tube. (j) Siliceous filament with discrete swellings (arrowed). (k) Curving filaments produced from iron sulfate seed grains in sodium carbonate solution. Scale bar: (a) 200 µm; (b) 95 µm; (c) 85 µm; (d) 45 µm; (e) 300 µm; (f) 115 µm; (g) 55 µm; (h) 55 µm; (i) 73 µm; (j) 70 µm; (k) 350 µm. (Online version in colour.)
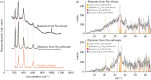
Figure 2.
Composition of chemical garden filaments showing iron (oxyhydr)oxides. (a) Raman spectra showing characteristic peaks for hematite obtained on repeat analysis of Raman laser-damaged filaments. The additional peaks at approximately 1000 cm−1 in the uppermost spectrum are due to the underlying plastic Petri dish. The hematite standard shown for comparison is RRUFF 040024 [37]. (b) XRD traces showing the occurrence of diffraction peaks at angles consistent with goethite, ferrihydrite, hematite, and feroxyhyte (corresponding reference sample numbers in the Crystallographic Open Database are indicated). The low signal-to-noise ratio is due to poor crystallinity and iron fluorescence. (Online version in colour.)
Figure 3.
Photomicrographs of individual filaments grown in sodium carbonate solutions acidified to pH 12–7. The filaments illustrated are of lengths 200 µm (pH 12), 120 µm (pH 11), 120 µm (pH 10), 220 µm (pH 9), 105 µm (pH 8), and 120 µm (pH 7). (Online version in colour.)
Similar articles
- A New Frontier for Palaeobiology: Earth's Vast Deep Biosphere.
McMahon S, Ivarsson M. McMahon S, et al. Bioessays. 2019 Aug;41(8):e1900052. doi: 10.1002/bies.201900052. Epub 2019 Jun 26. Bioessays. 2019. PMID: 31241200 Review. - Reassessing the biogenicity of Earth's oldest trace fossil with implications for biosignatures in the search for early life.
Grosch EG, McLoughlin N. Grosch EG, et al. Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):8380-5. doi: 10.1073/pnas.1402565111. Epub 2014 May 27. Proc Natl Acad Sci U S A. 2014. PMID: 24912193 Free PMC article. - Dubiofossils from a Mars-analogue subsurface palaeoenvironment: The limits of biogenicity criteria.
McMahon S, Ivarsson M, Wacey D, Saunders M, Belivanova V, Muirhead D, Knoll P, Steinbock O, Frost DA. McMahon S, et al. Geobiology. 2021 Sep;19(5):473-488. doi: 10.1111/gbi.12445. Epub 2021 May 5. Geobiology. 2021. PMID: 33951268 - Laser--Raman imagery of Earth's earliest fossils.
Schopf JW, Kudryavtsev AB, Agresti DG, Wdowiak TJ, Czaja AD. Schopf JW, et al. Nature. 2002 Mar 7;416(6876):73-6. doi: 10.1038/416073a. Nature. 2002. PMID: 11882894 - Paleo-Rock-Hosted Life on Earth and the Search on Mars: A Review and Strategy for Exploration.
Onstott TC, Ehlmann BL, Sapers H, Coleman M, Ivarsson M, Marlow JJ, Neubeck A, Niles P. Onstott TC, et al. Astrobiology. 2019 Oct;19(10):1230-1262. doi: 10.1089/ast.2018.1960. Epub 2019 Jun 25. Astrobiology. 2019. PMID: 31237436 Free PMC article. Review.
Cited by
- Molecular Profiling, Characterization and Antimicrobial Efficacy of Silver Nanoparticles Synthesized from Calvatia gigantea and Mycena leaiana against Multidrug-Resistant Pathogens.
Khan S, Fiaz M, Alvi IA, Ikram M, Yasmin H, Ahmad J, Ullah A, Niaz Z, Hayat S, Ahmad A, Kaushik P, Farid A. Khan S, et al. Molecules. 2023 Aug 28;28(17):6291. doi: 10.3390/molecules28176291. Molecules. 2023. PMID: 37687119 Free PMC article. - Hybrid organic-inorganic structures trigger the formation of primitive cell-like compartments.
Holler S, Bartlett S, Löffler RJG, Casiraghi F, Diaz CIS, Cartwright JHE, Hanczyc MM. Holler S, et al. Proc Natl Acad Sci U S A. 2023 Aug 15;120(33):e2300491120. doi: 10.1073/pnas.2300491120. Epub 2023 Aug 10. Proc Natl Acad Sci U S A. 2023. PMID: 37561785 Free PMC article. - An Alternative Approach for Assessing Biogenicity.
Rouillard J, van Zuilen M, Pisapia C, Garcia-Ruiz JM. Rouillard J, et al. Astrobiology. 2021 Feb;21(2):151-164. doi: 10.1089/ast.2020.2282. Epub 2020 Oct 13. Astrobiology. 2021. PMID: 33544651 Free PMC article. - A self-sustaining serpentinization mega-engine feeds the fougerite nanoengines implicated in the emergence of guided metabolism.
Russell MJ. Russell MJ. Front Microbiol. 2023 May 15;14:1145915. doi: 10.3389/fmicb.2023.1145915. eCollection 2023. Front Microbiol. 2023. PMID: 37275164 Free PMC article. - Metabolically diverse primordial microbial communities in Earth's oldest seafloor-hydrothermal jasper.
Papineau D, She Z, Dodd MS, Iacoviello F, Slack JF, Hauri E, Shearing P, Little CTS. Papineau D, et al. Sci Adv. 2022 Apr 15;8(15):eabm2296. doi: 10.1126/sciadv.abm2296. Epub 2022 Apr 13. Sci Adv. 2022. PMID: 35417227 Free PMC article.
References
- Little CT, Thorseth IH. 2002. Hydrothermal vent microbial communities: a fossil perspective. Cah. Biol. Mar. 43, 317–320.
- Grenne T, Slack JF. 2003. Bedded jaspers of the Ordovician Løkken ophiolite, Norway: seafloor deposition and diagenetic maturation of hydrothermal plume-derived silica-iron gels. Miner. Deposita 38, 625–639. (10.1007/s00126-003-0346-3) - DOI
- Little CT, Glynn SE, Mills RA. 2004. Four-hundred-and-ninety-million-year record of bacteriogenic iron oxide precipitation at sea-floor hydrothermal vents. Geomicrobiol. J. 21, 415–429. (10.1080/01490450490485845) - DOI
- Zhou X, Chen D, Tang D, Dong S, Guo C, Guo Z, Zhang Y. 2015. Biogenic iron-rich filaments in the quartz veins in the uppermost Ediacaran Qigebulake Formation, Aksu area, northwestern Tarim basin, China: implications for iron oxidizers in subseafloor hydrothermal systems. Astrobiology 15, 523–537. (10.1089/ast.2014.1234) - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous