Measles Virus Ribonucleoprotein Complexes Rapidly Spread across Well-Differentiated Primary Human Airway Epithelial Cells along F-Actin Rings - PubMed (original) (raw)
Measles Virus Ribonucleoprotein Complexes Rapidly Spread across Well-Differentiated Primary Human Airway Epithelial Cells along F-Actin Rings
Brajesh K Singh et al. mBio. 2019.
Abstract
Measles virus (MeV) is a highly contagious human pathogen that continues to be a worldwide health burden. One of the challenges for the study of MeV spread is the identification of model systems that accurately reflect how MeV behaves in humans. For our studies, we use unpassaged, well-differentiated primary cultures of airway epithelial cells from human donor lungs to examine MeV infection and spread. Here, we show that the main components of the MeV ribonucleoprotein complex (RNP), the nucleocapsid and phosphoprotein, colocalize with the apical and circumapical F-actin networks. To better understand how MeV infections spread across the airway epithelium, we generated a recombinant virus incorporating chimeric fluorescent proteins in its RNP complex. By live cell imaging, we observed rapid movement of RNPs along the circumapical F-actin rings of newly infected cells. This strikingly rapid mechanism of horizontal trafficking across epithelia is consistent with the opening of pores between columnar cells by the viral membrane fusion apparatus. Our work provides mechanistic insights into how MeV rapidly spreads through airway epithelial cells, contributing to its extremely contagious nature.IMPORTANCE The ability of viral particles to directly spread cell to cell within the airways without particle release is considered to be highly advantageous to many respiratory viruses. Our previous studies in well-differentiated, primary human airway epithelial cells suggest that measles virus (MeV) spreads cell to cell by eliciting the formation of intercellular membrane pores. Based on a newly generated ribonucleoprotein complex (RNP) "tracker" virus, we document by live-cell microscopy that MeV RNPs move along F-actin rings before entering a new cell. Thus, rather than diffusing through the cytoplasm of a newly infected columnar cell, RNPs take advantage of the cytoskeletal infrastructure to rapidly spread laterally across the human airway epithelium. This results in rapid horizontal spread through the epithelium that does not require particle release.
Keywords: actin; airways; lungs; measles; paramyxovirus.
Copyright © 2019 Singh et al.
Figures
FIG 1
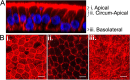
F-actin network in HAE cells. HAE cells were fixed and permeabilized, and F-actin filaments were visualized by staining with phalloidin (red) and nuclei by staining with DAPI (blue). Z-stacks were acquired by confocal microscopy. (A) The vertical section (xz) view from a z-stack series is shown. (B) The planes of the corresponding en face (xy) images are indicated. The F-actin networks in apical (i), circumapical (ii), and basolateral (iii) HAE cells are shown. As indicated, the en face views are comprised of maximum-intensity projection images of three to five z-stacks. Images are representative from n = 6 samples (two technical replicates from three human donors [biological replicates]). Scale bar, 20 μm.
FIG 2
Structure of F-actin within infectious centers. (A) Schematic of the MeV-GFP genome. The eGFP coding region was inserted between the leader sequence and the N gene as a separate transcription unit. (B) HAE cells were infected with MeV-GFP (MOI = 1) and imaged using confocal microscopy 72 h later. Fixed and permeabilized HAE cells were stained for F-actin with rhodamine-conjugated phalloidin (red), and the nuclei were visualized with DAPI (blue). Both en face (upper panels) and vertical (lower panels) sections are shown. Arrows indicate the central regions of the infectious center where F-actin has disassembled. Scale bar, 20 μm. Images are representative from n = 6 samples (two technical replicates from three human donors [biological replicates]). (C) HAE cells were infected with MeV-GFP at an MOI of 1 and, 36 h later, the cultures were treated with the F-actin-disrupting agents cytochalasin D (CytoD), CK-666, or SMIFH2 or a control (DMSO) for 24 h. The areas of the infectious centers were quantified using ImageJ software at the time of drug delivery (36 h) and 24 h after drug delivery (60 h). Each dot represents the log-transformed value of the area of individual infectious centers. The data for each condition are pooled from six human donors. AU, arbitrary unit. Adjusted P value using one-way ANOVA corrected for multiple comparisons were determined (***, P < 0.001; ****, P < 0.0001).
FIG 3
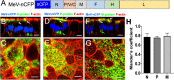
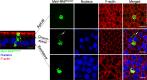
Localization of MeV N, P, and M proteins within infectious centers. (A) Schematic of the MeV-nCFP genome. The coding region of nuclear-targeted cyan fluorescent protein (nCFP) was inserted between the leader sequence and the N gene as a separate transcription unit. (B to G) Images of N, P, and M protein localization. HAE cells were infected with MeV-nCFP (blue) and, at 72 hpi, the cells were fixed and immunostained for N protein (B and C), P protein (D and E), or M protein (F and G) (green). F-actin was stained with phalloidin (red). The cells were examined by confocal microscopy. White arrows indicate apical localization, and green arrows indicate perinuclear localization. Vertical sections of immunostained cultures are shown in panels B, D, and F. Scale bar, 20 μm. Cells were then examined by stimulated emission depletion (STED) superresolution microscopy (C, E, and G). En face views of immunostained cultures are shown. Scale bar, 5 μm. Dotted lines in panels B, D, and F indicate the approximate plane of view for panels C, E, and G, respectively. Images are representative from n = 9 samples (three technical replicates from three human donors [biological replicates]). (H) Quantification of colocalization between viral proteins (N, P, and M) and F-actin at the apical surface in infectious centers was measured by applying Mander’s colocalization coefficient using Coloc2 plugin in Fiji.
FIG 4
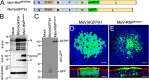
Generation and characterization of MeV-RNPtracker. (A) Schematics of the MeV-RNPtracker genome (top) and of the MeV(GFP)H genome (bottom) control virus. In MeV-RNPtracker, GFP was fused in frame with a second copy of the P protein (GFP/P), and the transcription unit was inserted between the H and L genes. In MeV(GFP)H, a transcription unit expressing GFP was inserted in the same position. (B and C) Immunoblot characterization of the P proteins expressed by the two viruses. In panel B, the expression of the N (top), P and GFP/P (center), or control actin (bottom) proteins was analyzed. In panel C, the expression of GFP/P and GFP proteins were analyzed. At 3 days postinfection, the GFP expression of MeV(GFP)H (D) and MeV-RNPtracker (E) was examined by confocal microscopy. Nuclei were stained with DAPI (blue), and F-actin was visualized with phalloidin (red, only shown in the bottom panel vertical sections). Images are representative from n = 6 samples (two technical replicates from three human donors [biological replicates]). Scale bars, 20 μm.
FIG 5
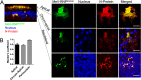
Intracellular distribution of RNPs in RNPtracker infected HAE. (A) Cells were infected with MeV-RNPtracker and imaged at 72 hpi by confocal microscopy. The cells were fixed, permeabilized, and then immunostained for N protein (red). Nuclei were visualized with DAPI (blue). The left panel is a vertical section view, and the vertical bars indicate the plane of view for the series of en face images on the right. The right panels show maximum intensity projection images of three to five z-stacks at the indicated apical, circumapical, and basolateral regions. Scale bars, 20 μm. Images are representative from n = 6 samples (two technical replicates from three human donors [biological replicates]). (B) Colocalization between RNPtracker and N protein within infectious centers was measured by using Mander’s colocalization coefficient.
FIG 6
In newly infected cells, MeV RNPs localize to the circum-apical network. Cells were infected with MeV-RNPtracker, fixed, and imaged at 36 hpi by confocal microscopy. Cells were counterstained for F-actin with phalloidin (red), and nuclei were visualized with DAPI (blue). The left panel is a vertical section view, and the vertical bars indicate the plane of view for the series of en face images on the right. The right panels show maximum intensity projection images of three to five z-stacks at the indicated apical, circumapical, and basolateral regions. White arrows indicate MeV RNPs along the circumapical region of the F-actin network in newly infected cells. Images are representative from n = 6 samples (two technical replicates from three human donors [biological replicates]). Scale bars, 10 μm.
FIG 7
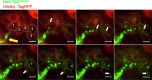
Time-lapse microscopy analysis of MeV RNPs transport along the F-actin network. HAE cells were transduced with an adenoviral vector expressing LifeAct-TagRFP (red). After 24 h, HAE cells were infected with MeV-RNPtracker (green). Fluorescence was monitored by confocal time-lapse microscopy beginning at 48 h after MeV infection; the spread of MeV-RNPs is shown at approximately 2-h intervals. The yellow arrow indicates the initial infected cell. Dotted lines in the first panel indicate individual columnar epithelial cells, as well as the circumapical F-actin networks. The cells are numbered 1 to 5 from nearest to furthest from the initial infected cell. White arrows indicate the unidirectional flow of MeV RNPs. Images correspond to Video S4. Scale bars, 10 μm.
Similar articles
- Cell-to-Cell Contact and Nectin-4 Govern Spread of Measles Virus from Primary Human Myeloid Cells to Primary Human Airway Epithelial Cells.
Singh BK, Li N, Mark AC, Mateo M, Cattaneo R, Sinn PL. Singh BK, et al. J Virol. 2016 Jul 11;90(15):6808-6817. doi: 10.1128/JVI.00266-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27194761 Free PMC article. - The Nectin-4/Afadin Protein Complex and Intercellular Membrane Pores Contribute to Rapid Spread of Measles Virus in Primary Human Airway Epithelia.
Singh BK, Hornick AL, Krishnamurthy S, Locke AC, Mendoza CA, Mateo M, Miller-Hunt CL, Cattaneo R, Sinn PL. Singh BK, et al. J Virol. 2015 Jul;89(14):7089-96. doi: 10.1128/JVI.00821-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926640 Free PMC article. - Epithelial-to-mesenchymal transition and live cell extrusion contribute to measles virus release from human airway epithelia.
Hippee CE, Durnell LA, Kaufman JW, Murray E, Singh BK, Sinn PL. Hippee CE, et al. J Virol. 2025 Feb 25;99(2):e0122024. doi: 10.1128/jvi.01220-24. Epub 2025 Jan 10. J Virol. 2025. PMID: 39791903 Free PMC article. - Stronger together: Multi-genome transmission of measles virus.
Cattaneo R, Donohue RC, Generous AR, Navaratnarajah CK, Pfaller CK. Cattaneo R, et al. Virus Res. 2019 May;265:74-79. doi: 10.1016/j.virusres.2019.03.007. Epub 2019 Mar 7. Virus Res. 2019. PMID: 30853585 Free PMC article. Review. - The measles virus replication cycle.
Rima BK, Duprex WP. Rima BK, et al. Curr Top Microbiol Immunol. 2009;329:77-102. doi: 10.1007/978-3-540-70523-9_5. Curr Top Microbiol Immunol. 2009. PMID: 19198563 Review.
Cited by
- Viral cell-to-cell spread: Conventional and non-conventional ways.
Cifuentes-Munoz N, El Najjar F, Dutch RE. Cifuentes-Munoz N, et al. Adv Virus Res. 2020;108:85-125. doi: 10.1016/bs.aivir.2020.09.002. Epub 2020 Sep 29. Adv Virus Res. 2020. PMID: 33837723 Free PMC article. Review. - Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies.
Kinder JT, Moncman CL, Barrett C, Jin H, Kallewaard N, Dutch RE. Kinder JT, et al. J Virol. 2020 Sep 29;94(20):e01068-20. doi: 10.1128/JVI.01068-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32759319 Free PMC article. - Vectorial Release of Human RNA Viruses from Epithelial Cells.
Chapuy-Regaud S, Allioux C, Capelli N, Migueres M, Lhomme S, Izopet J. Chapuy-Regaud S, et al. Viruses. 2022 Jan 25;14(2):231. doi: 10.3390/v14020231. Viruses. 2022. PMID: 35215825 Free PMC article. Review. - The contributions of T cell-mediated immunity to protection from vaccine-preventable diseases: A primer.
Shapiro JR, Corrado M, Perry J, Watts TH, Bolotin S. Shapiro JR, et al. Hum Vaccin Immunother. 2024 Dec 31;20(1):2395679. doi: 10.1080/21645515.2024.2395679. Epub 2024 Aug 29. Hum Vaccin Immunother. 2024. PMID: 39205626 Free PMC article. Review. - Noncanonical Transmission of a Measles Virus Vaccine Strain from Neurons to Astrocytes.
Poelaert KCK, Williams RM, Matullo CM, Rall GF. Poelaert KCK, et al. mBio. 2021 Mar 23;12(2):e00288-21. doi: 10.1128/mBio.00288-21. mBio. 2021. PMID: 33758092 Free PMC article.
References
- Lamb RA, Parks G. 2013. Paramyxoviridae, p 957–995. In Knipe DM, Howley PM (ed), Fields virology. Lippincott/Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical