Diabetes, a Contemporary Risk for Parkinson's Disease: Epidemiological and Cellular Evidences - PubMed (original) (raw)
Diabetes, a Contemporary Risk for Parkinson's Disease: Epidemiological and Cellular Evidences
Domenico Sergi et al. Front Aging Neurosci. 2019.
Abstract
Diabetes mellitus (DM), a group of diseases characterized by defective glucose metabolism, is the most widespread metabolic disorder affecting over 400 million adults worldwide. This pathological condition has been implicated in the pathogenesis of a number of central encephalopathies and peripheral neuropathies. In further support of this notion, recent epidemiological evidence suggests a link between DM and Parkinson's disease (PD), with hyperglycemia emerging as one of the culprits in neurodegeneration involving the nigrostriatal pathway, the neuroanatomical substrate of the motor symptoms affecting parkinsonian patients. Indeed, dopaminergic neurons located in the mesencephalic substantia nigra appear to be particularly vulnerable to oxidative stress and degeneration, likely because of their intrinsic susceptibility to mitochondrial dysfunction, which may represent a direct consequence of hyperglycemia and hyperglycemia-induced oxidative stress. Other pathological pathways induced by increased intracellular glucose levels, including the polyol and the hexosamine pathway as well as the formation of advanced glycation end-products, may all play a pivotal role in mediating the detrimental effects of hyperglycemia on nigral dopaminergic neurons. In this review article, we will examine the epidemiological as well as the molecular and cellular clues supporting the potential susceptibility of nigrostriatal dopaminergic neurons to hyperglycemia.
Keywords: dopamine; glucotoxicity; hyperglycemia; mitochondrial dysfunction; nigrostriatal pathway; oxidative stress.
Copyright © 2019 Sergi, Renaud, Simola and Martinoli.
Figures
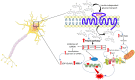
Figure 1
Mechanisms of neuronal glucotoxicity. In the early stages of hyperglycemia, there is an upregulation of glucose flux through glycolysis and the tricarboxylic acid cycle (TCA). This results in enhanced electron donor supply to the mitochondrial electron transport chain, leading to an increase in the proton gradient across the mitochondrial inner membrane which promotes the formation of reactive oxygen species (ROS). ROS-induced DNA damage triggers the activation of the NAD+-dependent DNA repair enzyme, poly (adenosine diphosphate-ribose) polymerase 1 (PARP1), responsible for depleting neuronal NAD+. The increase in intracellular glucose activates the polyol pathway which, in concert with PARP1, reduces intracellular NAD+ inhibiting glyceraldehyde triphosphate dehydrogenase (GAPDH) and therefore glycolysis. The inhibition of GAPDH provokes the accumulation of upstream triose phosphates then converted to glycating agents, thereby promoting the formation of advanced glycation end products (AGEs). The accumulation of fructose, another metabolite upstream GAPDH, triggers the activation of the hexosamine pathway and the subsequent build-up of UDP-N-acetylglucosamine (UDP-GlcNAc). Furthermore, the activation of the polyol pathway depletes NADPH and, alongside mitochondrial ROS, contributes to oxidative stress in light of its role as a cofactor for glutathione reductase, which, in turn, is crucial in maintaining the intracellular pool of reduced glutathione.
Similar articles
- Dopaminergic neurodegeneration in a rat model of long-term hyperglycemia: preferential degeneration of the nigrostriatal motor pathway.
Renaud J, Bassareo V, Beaulieu J, Pinna A, Schlich M, Lavoie C, Murtas D, Simola N, Martinoli MG. Renaud J, et al. Neurobiol Aging. 2018 Sep;69:117-128. doi: 10.1016/j.neurobiolaging.2018.05.010. Epub 2018 May 14. Neurobiol Aging. 2018. PMID: 29890391 - Diabetes and Cognitive Impairment: A Role for Glucotoxicity and Dopaminergic Dysfunction.
Pignalosa FC, Desiderio A, Mirra P, Nigro C, Perruolo G, Ulianich L, Formisano P, Beguinot F, Miele C, Napoli R, Fiory F. Pignalosa FC, et al. Int J Mol Sci. 2021 Nov 16;22(22):12366. doi: 10.3390/ijms222212366. Int J Mol Sci. 2021. PMID: 34830246 Free PMC article. Review. - Neuroprotective effect of dextromethorphan in the MPTP Parkinson's disease model: role of NADPH oxidase.
Zhang W, Wang T, Qin L, Gao HM, Wilson B, Ali SF, Zhang W, Hong JS, Liu B. Zhang W, et al. FASEB J. 2004 Mar;18(3):589-91. doi: 10.1096/fj.03-0983fje. Epub 2004 Jan 20. FASEB J. 2004. PMID: 14734632 - Linking Glycation and Glycosylation With Inflammation and Mitochondrial Dysfunction in Parkinson's Disease.
Videira PAQ, Castro-Caldas M. Videira PAQ, et al. Front Neurosci. 2018 Jun 7;12:381. doi: 10.3389/fnins.2018.00381. eCollection 2018. Front Neurosci. 2018. PMID: 29930494 Free PMC article. Review.
Cited by
- Protective properties of GLP-1 and associated peptide hormones in neurodegenerative disorders.
Hölscher C. Hölscher C. Br J Pharmacol. 2022 Feb;179(4):695-714. doi: 10.1111/bph.15508. Epub 2021 May 29. Br J Pharmacol. 2022. PMID: 33900631 Free PMC article. Review. - Influence of Type 2 Diabetes and Adipose Tissue Dysfunction on Breast Cancer and Potential Benefits from Nutraceuticals Inducible in Microalgae.
Sergi D, Melloni M, Passaro A, Neri LM. Sergi D, et al. Nutrients. 2024 Sep 25;16(19):3243. doi: 10.3390/nu16193243. Nutrients. 2024. PMID: 39408212 Free PMC article. Review. - Ferroptosis-A Shared Mechanism for Parkinson's Disease and Type 2 Diabetes.
Duță C, Muscurel C, Dogaru CB, Stoian I. Duță C, et al. Int J Mol Sci. 2024 Aug 14;25(16):8838. doi: 10.3390/ijms25168838. Int J Mol Sci. 2024. PMID: 39201524 Free PMC article. Review. - Multifactoriality of Parkinson's Disease as Explored Through Human Neural Stem Cells and Their Transplantation in Middle-Aged Parkinsonian Mice.
Nelke A, García-López S, Martínez-Serrano A, Pereira MP. Nelke A, et al. Front Pharmacol. 2022 Jan 19;12:773925. doi: 10.3389/fphar.2021.773925. eCollection 2021. Front Pharmacol. 2022. PMID: 35126116 Free PMC article. - Failure of diet-induced transcriptional adaptations in alpha-synuclein transgenic mice.
Kilzheimer A, Hentrich T, Rotermund C, Kahle PJ, Schulze-Hentrich JM. Kilzheimer A, et al. Hum Mol Genet. 2023 Jan 13;32(3):450-461. doi: 10.1093/hmg/ddac205. Hum Mol Genet. 2023. PMID: 36001352 Free PMC article.
References
- Akude E., Zherebitskaya E., Chowdhury S. K., Smith D. R., Dobrowsky R. T., Fernyhough P. (2011). Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes 60, 288–297. 10.2337/db10-0818 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources