Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis For Wound Healing Applications - PubMed (original) (raw)
Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis For Wound Healing Applications
Syed Raza Ur Rehman et al. Int J Nanomedicine. 2019.
Erratum in
- Erratum: Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis for Wound Healing Applications [Corrigendum].
[No authors listed] [No authors listed] Int J Nanomedicine. 2022 Jun 14;17:2643-2645. doi: 10.2147/IJN.S367492. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35726213 Free PMC article. - Erratum: Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis for Wound Healing Applications [Corrigendum].
[No authors listed] [No authors listed] Int J Nanomedicine. 2022 Aug 15;17:3601-3602. doi: 10.2147/IJN.S381536. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35996528 Free PMC article.
Abstract
Purpose: Non-healing or slow healing chronic wounds are among serious complications of diabetes that eventually result in amputation of limbs and increased morbidities and mortalities. Chronic diabetic wounds show reduced blood vessel formation (lack of angiogenesis), inadequate cell proliferation and poor cell migration near wounds. In this paper, we report the development of a hydrogel-based novel wound dressing material loaded with reduced graphene oxide (rGO) to promote cell proliferation, cell migration and angiogenesis for wound healing applications.
Methods: Gelatin-methacryloyl (GelMA) based hydrogels loaded with different concentrations of rGO were fabricated by UV crosslinking. Morphological and physical characterizations (porosity, degradation, and swelling) of rGO incorporated GelMA hydrogel was performed. In vitro cell proliferation, cell viability and cell migration potential of the hydrogels were analyzed by MTT assay, live/dead staining, and wound healing scratch assay respectively. Finally, in vivo chicken embryo angiogenesis (CEO) testing was performed to evaluate the angiogenic potential of the prepared hydrogel.
Results: The experimental results showed that the developed hydrogel possessed enough porosity and exudate-absorbing capacity. The biocompatibility of prepared hydrogel on three different cell lines (3T3 fibroblasts, EA.hy926 endothelial cells, and HaCaT keratinocytes) was confirmed by in vitro cell culture studies (live/dead assay). The GelMA hydrogel containing 0.002% w/w rGO considerably increased the proliferation and migration of cells as evident from MTT assay and wound healing scratch assay. Furthermore, rGO impregnated GelMA hydrogel significantly enhanced the angiogenesis in the chick embryo model.
Conclusion: The positive effect of 0.002% w/w rGO impregnated GelMA hydrogels on angiogenesis, cell migration and cell proliferation suggests that these formulations could be used as a functional wound healing material for the healing of chronic wounds.
Keywords: GelMA hydrogel; angiogenesis; nanocomposite hydrogel; reduced graphene oxide; wound healing.
© 2019 Rehman et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures
Figure 1
Schematic representation for the fabrication of nanocomposite GelMA hydrogel by UV crosslinking method.
Figure 2
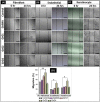
SEM micrograph at 10000X magnification of the surface of (A) GelMA hydrogel, (B) Cross-sectional image of GelMA hydrogel at 2500X, & (C) Cross-sectional image of GelMA hydrogel at 5000X magnification. (D) XRD curves of blank GelMA hydrogel, rGO nanoparticles, 0.001 wt% rGO loaded GelMA hydrogel (GrG1), 0.002 wt% rGO loaded GelMA hydrogel (GrG2) respectively.
Figure 3
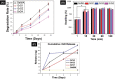
(A) Degradation curve of blank GelMA hydrogel and nanocomposite hydrogels with respect to time. Error bars represent the SD of measurements performed on at least 5 specimens. (B) The swelling percentage of blank GelMA hydrogel and nanocomposite GelMA hydrogel after 0, 15, 30, 60 and 120 mins respectively (*P <0.05). (C) Cumulative Release of rGO from the nanocomposite GelMA hydrogel (GrG1, GrG2, and GrG4) and blank GelMA hydrogel after 1, 3, and 5 days in PBS.
Figure 4
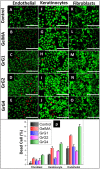
(A–E) Cell viability (Live/Dead assay) on Endothelial cells, (F–J) 3T3 fibroblast cells and (K–O) HaCat keratinocyte cells for control, blank GelMA hydrogel, 0.001 wt% rGO loaded GelMA hydrogel (GrG1), 0.002 wt% rGO loaded GelMA hydrogel (GrG2) and 0.004 wt% rGO loaded GelMA hydrogel (GrG4) respectively. Green channel depicts live cells, while red channels depict dead cells. (P) Quantitative comparison of the percentage of dead cells. The scale bar at the right lower corner is 1000 µm.
Figure 5
The histogram of MTT assay, comparing the proliferation of (A) endothelial cells, (B) 3T3 fibroblasts, and (C) HaCat keratinocytes after 1 day, 3 days, and 5 days of incubations. by the treatment of blank GelMA hydrogel, 0.001 wt% rGO loaded GelMA hydrogel (GrG1), 0.002 wt% rGO loaded GelMA hydrogel (GrG2) and 0.004 wt% rGO loaded GelMA hydrogel (GrG4) and control (untreated). (*P < 0.05, **P < 0.02).
Figure 6
Results of wound healing scratch assay using (A) 3T3 fibroblast cells, (B) Endothelial Cells, (C) HaCat Keratinocyte cells for control (untreated), blank GelMA hydrogel, 0.001 wt% rGO loaded GelMA hydrogel (GrG1), 0.002 wt% rGO loaded GelMA hydrogel (GrG2), & 0.004% rGO loaded GelMA hydrogel (GrG4) treated cells. (D) Percentage of wound healing was measured and presented on a histogram using ImageJ software. (*P < 0.05, **P < 0.01). The scale bar at the right lower corner is 1000 µm.
Figure 7
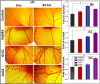
(A) Results of i_n vivo_ CEA assay of control (untreated samples), in the presence of the blank GelMA hydrogel, 0.001 wt% rGO loaded GelMA hydrogel (GrG1), and 0.002 wt% rGO loaded GelMA hydrogel (GrG2). Increase of matured blood vessel formation (marked by black arrows) was observed in embryo treated with GelMA hydrogel with 0.002 wt% rGO nanoparticles (GrG2). Several angiogenic parameters such as blood vessel junction, length, and thickness were quantified and presented as a histogram (B–D respectively). Statistical significance was calculated by using t -test. All data are statistically significant (*P < 0.05).
Similar articles
- [Effects of in situ cross-linked graphene oxide-containing gelatin methacrylate anhydride hydrogel on wound vascularization of full-thickness skin defect in mice].
Liang LT, Song W, Zhang C, Li Z, Yao B, Zhang MD, Yuan XY, Jirigala E, Fu XB, Huang S, Zhu P. Liang LT, et al. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022 Jul 20;38(7):616-628. doi: 10.3760/cma.j.cn501225-20220314-00063. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022. PMID: 35899412 Free PMC article. Chinese. - A multi-purpose dressing based on resveratrol-loaded ionic liquids/gelatin methacryloyl hydrogel for enhancing diabetic wound healing.
Xu S, Jiang C, Yu T, Chen K. Xu S, et al. Int J Biol Macromol. 2024 Dec;283(Pt 2):136773. doi: 10.1016/j.ijbiomac.2024.136773. Epub 2024 Oct 21. Int J Biol Macromol. 2024. PMID: 39442835 - VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis.
Wang Y, Cao Z, Wei Q, Ma K, Hu W, Huang Q, Su J, Li H, Zhang C, Fu X. Wang Y, et al. Acta Biomater. 2022 Jul 15;147:342-355. doi: 10.1016/j.actbio.2022.05.018. Epub 2022 May 16. Acta Biomater. 2022. PMID: 35580827 - Recent Advances on Bioprinted Gelatin Methacrylate-Based Hydrogels for Tissue Repair.
Rajabi N, Rezaei A, Kharaziha M, Bakhsheshi-Rad HR, Luo H, RamaKrishna S, Berto F. Rajabi N, et al. Tissue Eng Part A. 2021 Jun;27(11-12):679-702. doi: 10.1089/ten.TEA.2020.0350. Epub 2021 Mar 9. Tissue Eng Part A. 2021. PMID: 33499750 Review. - Recent advances on 3D-bioprinted gelatin methacrylate hydrogels for tissue engineering in wound healing: A review of current applications and future prospects.
Wang H, Wan J, Zhang Z, Hou R. Wang H, et al. Int Wound J. 2024 Apr;21(4):e14533. doi: 10.1111/iwj.14533. Epub 2023 Dec 9. Int Wound J. 2024. PMID: 38069620 Free PMC article. Review.
Cited by
- The Design and Characterization of a Strong Bio-Ink for Meniscus Regeneration.
Lu J, Huang J, Jin J, Xie C, Xue B, Lai J, Cheng B, Li L, Jiang Q. Lu J, et al. Int J Bioprint. 2022 Aug 8;8(4):600. doi: 10.18063/ijb.v8i4.600. eCollection 2022. Int J Bioprint. 2022. PMID: 36483752 Free PMC article. - Cadherin-responsive hydrogel combined with dental pulp stem cells and fibroblast growth factor 21 promotes diabetic scald repair via regulating epithelial-mesenchymal transition and necroptosis.
Lu W, Zhao J, Cai X, Wang Y, Lin W, Fang Y, Wang Y, Ao J, Shou J, Xu J, Zhu S. Lu W, et al. Mater Today Bio. 2023 Dec 22;24:100919. doi: 10.1016/j.mtbio.2023.100919. eCollection 2024 Feb. Mater Today Bio. 2023. PMID: 38298888 Free PMC article. - Encapsulation of Thymol in Gelatin Methacryloyl (GelMa)-Based Nanoniosome Enables Enhanced Antibiofilm Activity and Wound Healing.
Moghtaderi M, Bazzazan S, Sorourian G, Sorourian M, Akhavanzanjani Y, Noorbazargan H, Ren Q. Moghtaderi M, et al. Pharmaceutics. 2023 Jun 9;15(6):1699. doi: 10.3390/pharmaceutics15061699. Pharmaceutics. 2023. PMID: 37376147 Free PMC article. - Light-controlled growth factors release on tetrapodal ZnO-incorporated 3D-printed hydrogels for developing smart wound scaffold.
Siebert L, Luna-Cerón E, García-Rivera LE, Oh J, Jang J, Rosas-Gómez DA, Pérez-Gómez MD, Maschkowitz G, Fickenscher H, Oceguera-Cuevas D, Holguín-León CG, Byambaa B, Hussain MA, Enciso-Martinez E, Cho M, Lee Y, Sobahi N, Hasan A, Orgill DP, Mishra YK, Adelung R, Lee E, Shin SR. Siebert L, et al. Adv Funct Mater. 2021 May 26;31(22):2007555. doi: 10.1002/adfm.202007555. Epub 2021 Feb 19. Adv Funct Mater. 2021. PMID: 36213489 Free PMC article. - Human Mesenchymal Stem Cells on Size-Sorted Gelatin Hydrogel Microparticles Show Enhanced In Vitro Wound Healing Activities.
Ozhava D, Bektas C, Lee K, Jackson A, Mao Y. Ozhava D, et al. Gels. 2024 Jan 26;10(2):97. doi: 10.3390/gels10020097. Gels. 2024. PMID: 38391427 Free PMC article.
References
- Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. Diabetes Am. 1995;2:409–427.
- Augustine R, Hasan A, Yadu Nath VK, et al. Electrospun polyvinyl alcohol membranes incorporated with green synthesized silver nanoparticles for wound dressing applications. J Mat Sci. 2018;29(11):163. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources