Omega-3 Fatty Acid Supplementation Reduces Intervertebral Disc Degeneration - PubMed (original) (raw)
Omega-3 Fatty Acid Supplementation Reduces Intervertebral Disc Degeneration
Zachary NaPier et al. Med Sci Monit. 2019.
Abstract
BACKGROUND Intervertebral disc (IVD) degeneration is a common cause of lower back pain, which carries substantial morbidity and economic cost. Omega-3 fatty acids (n-3 FA) are known to reduce inflammatory processes with a relatively benign side effect profile. This study aimed to investigate the effect of n-3 FA supplementation on IVD degeneration. MATERIAL AND METHODS Two non-contiguous lumbar discs of 12 Sprague Dawley rats were needle-punctured to induce disc degeneration. Post-surgery, rats were randomly assigned to either a daily n-3 FA diet (530 mg/kg/day of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in a 2: 1 ratio, administered in sucrose solution) or control diet (sucrose solution only), which was given for the duration of the study. After 1 month, blood serum arachidonic acid/eicosapentaenoic acid (AA/EPA) ratios were analyzed. After 2 months, micro-MRI (magnetic resonance imaging) analysis and histological staining of disc explants were performed to analyze the IVD. RESULTS A reduction of blood AA/EPA ratios from 40 to 20 was demonstrated after 1 month of daily supplementation with n-3 FA. Micro-MRI analysis showed an injury-induced reduction of IVD hydration, which was attenuated in rats receiving n-3 FA. Histological evaluation demonstrated the destruction of nucleus pulposus tissue in response to needle puncture injury, which was less severe in the n-3 FA diet group. CONCLUSIONS The results of this study suggest that n-3 FA dietary supplementation reduces systemic inflammation by lowering AA/EPA ratios in blood serum and has potential protective effects on the progression of spinal disc degeneration, as demonstrated by reduced needle injury-induced dehydration of intervertebral discs and reduced histological signs of IVD degeneration.
Figures
Figure 1
Study design. Left is a radiographic image of a rat lumbar spine. The red arrows indicate the sites of disc needle injury. Middle is anterior retroperitoneal approach to the lumbar spine with exposure of 3 consecutive discs with 18-gauge needle puncture of middle IVD. Right is post-surgery: rats were assigned to either the control (oral sucrose solution) group or n-3 FA diet group. Diet supplementation occurred daily for the duration of the experiment. AA/EPA blood and micro-MRI analyses were performed in vivo. Post-surgery, histological analysis was performed. Sac – day of sacrifice; IVD – intervertebral disc; n-3 FA – omega-3 fatty acids; AA/EPA – arachidonic acid/eicosapentaenoic acid; MRI – magnetic resonance imaging.
Figure 2
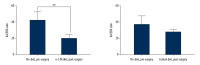
Reduction in serum AA/EPA ratio between pre-surgical and 4 weeks post-surgical rats receiving n-3 FA diet. Graphs show AA/EPA ratios tested in blood samples that were obtained pre-surgery, and after surgery in the n-3 FA diet group versus control diet group. n=4 per group. AA/EPA – arachidonic acid/eicosapentaenoic acid; n-3 FA – omega-3 fatty acids; MRI – magnetic resonance imaging.
Figure 3
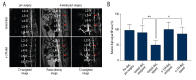
Injury-induced reduction in disc hydration is attenuated in rats receiving n-3 FA diet. (A) Representative T2-weighted and proton density images of the n-3 FA diet and control diet groups pre- and 1-month post-surgery. (B) Shows relative high signal NP area values of T2-weighted images. Data were normalized to pre-surgery/pre-diet scans. n=≥6 per group. n-3 FA – omega-3 fatty acids; NP – nucleus pulposus.
Figure 4
Destruction of nucleus pulposus in response to needle puncture injury is decreased in the n-3 FA diet group compared to control diet group. Shown are H&E staining of injured and uninjured rat lumbar discs from both groups at 2 months post-surgery. n-3 FA – omega-3 fatty acids; H&E – hematoxylin and eosin.
Similar articles
- NF-κB inhibitor, NEMO-binding domain peptide attenuates intervertebral disc degeneration.
Glaeser JD, Salehi K, Kanim LEA, NaPier Z, Kropf MA, Cuéllar JM, Perry TG, Bae HW, Sheyn D. Glaeser JD, et al. Spine J. 2020 Sep;20(9):1480-1491. doi: 10.1016/j.spinee.2020.04.025. Epub 2020 May 12. Spine J. 2020. PMID: 32413485 Free PMC article. - Selection of the Optimal Puncture Needle for Induction of a Rat Intervertebral Disc Degeneration Model.
Qian J, Ge J, Yan Q, Wu C, Yang H, Zou J. Qian J, et al. Pain Physician. 2019 Jul;22(4):353-360. Pain Physician. 2019. PMID: 31337166 - The dual effects of iron deficiency on intervertebral disc development and on acute injury-induced disc degeneration.
Chang H, Kang S, Li C, Wu L, Liu H, Zhang D, Chang Y. Chang H, et al. Sci Rep. 2025 Jun 2;15(1):19323. doi: 10.1038/s41598-025-04388-4. Sci Rep. 2025. PMID: 40456894 Free PMC article. - Role of Advanced Glycation End Products in Intervertebral Disc Degeneration: Mechanism and Therapeutic Potential.
Yang F, Zhu D, Wang Z, Ma Y, Huang L, Kang X, Ma B. Yang F, et al. Oxid Med Cell Longev. 2022 Dec 17;2022:7299005. doi: 10.1155/2022/7299005. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36573114 Free PMC article. Review. - Gut-disc axis: A cause of intervertebral disc degeneration and low back pain?
Li W, Lai K, Chopra N, Zheng Z, Das A, Diwan AD. Li W, et al. Eur Spine J. 2022 Apr;31(4):917-925. doi: 10.1007/s00586-022-07152-8. Epub 2022 Mar 14. Eur Spine J. 2022. PMID: 35286474 Review.
Cited by
- Optimization of a rat lumbar IVD degeneration model for low back pain.
Glaeser JD, Tawackoli W, Ju DG, Yang JH, Kanim LE, Salehi K, Yu V, Saidara E, Vit JP, Khnkoyan Z, NaPier Z, Stone LS, Bae HW, Sheyn D. Glaeser JD, et al. JOR Spine. 2020 Jun 22;3(2):e1092. doi: 10.1002/jsp2.1092. eCollection 2020 Jun. JOR Spine. 2020. PMID: 32613167 Free PMC article. - Intervertebral Disc Diseases PART 2: A Review of the Current Diagnostic and Treatment Strategies for Intervertebral Disc Disease.
Wu PH, Kim HS, Jang IT. Wu PH, et al. Int J Mol Sci. 2020 Mar 20;21(6):2135. doi: 10.3390/ijms21062135. Int J Mol Sci. 2020. PMID: 32244936 Free PMC article. Review. - Association between dietary inflammatory index and musculoskeletal disorders in adults.
Khamoushi F, Soleimani D, Najafi F, Ahmadi N, Heidarzadeh-Esfahani N, Anvari B, Shakiba E, Pasdar Y. Khamoushi F, et al. Sci Rep. 2023 Nov 20;13(1):20302. doi: 10.1038/s41598-023-46429-w. Sci Rep. 2023. PMID: 37985726 Free PMC article. - Intervertebral disc human nucleus pulposus cells associated with back pain trigger neurite outgrowth in vitro and pain behaviors in rats.
Jiang W, Glaeser JD, Kaneda G, Sheyn J, Wechsler JT, Stephan S, Salehi K, Chan JL, Tawackoli W, Avalos P, Johnson C, Castaneda C, Kanim LEA, Tanasansomboon T, Burda JE, Shelest O, Yameen H, Perry TG, Kropf M, Cuellar JM, Seliktar D, Bae HW, Stone LS, Sheyn D. Jiang W, et al. Sci Transl Med. 2023 Dec 6;15(725):eadg7020. doi: 10.1126/scitranslmed.adg7020. Epub 2023 Dec 6. Sci Transl Med. 2023. PMID: 38055799 Free PMC article. - n-3 polyunsaturated fatty acids delay intervertebral disc degeneration by inhibiting nuclear receptor coactivator 4-mediated iron overload.
Ao X, Jiang T, Li Y, Lai W, Lian Z, Wang L, Huang M, Zhang Z. Ao X, et al. iScience. 2023 Dec 13;27(2):108721. doi: 10.1016/j.isci.2023.108721. eCollection 2024 Feb 16. iScience. 2023. PMID: 38303704 Free PMC article.
References
- Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: Estimates from US national surveys 2002. Spine. 2006;31:2724–27. - PubMed
- Yang S-H, Hu M-H, Sun Y-H, Lin F-H. Differential phenotypic behaviors of human degenerative nucleus pulposus cells under normoxic and hypoxic conditions: influence of oxygen concentration during isolation, expansion, and cultivation. Spine J. 2013;13:1590–96. - PubMed
- Hilario MO, Terreri MT, Len CA. Nonsteroidal anti-inflammatory drugs: Cyclooxygenase 2 inhibitors. J Pediatr (Rio J) 2006;82:S206–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials