Sea anemone and clownfish microbiota diversity and variation during the initial steps of symbiosis - PubMed (original) (raw)
Sea anemone and clownfish microbiota diversity and variation during the initial steps of symbiosis
Natacha Roux et al. Sci Rep. 2019.
Abstract
Clownfishes and sea anemones form an intriguing long-term association, but the mechanism underlying this symbiosis is not well understood. Since clownfishes seem to cover themselves with sea anemone mucus, we investigated the microbiomes of the two partners to search for possible shifts in their compositions. We used a 16S rRNA gene sequencing strategy to study the dynamics of the microbiota during the association between the clownfish Amphiprion ocellaris and its host Heteractis magnifica under laboratory conditions. The experiment conducted in aquaria revealed that both clownfish and sea anemone mucus had specific signatures compared to artificial sea water. The microbiomes of both species were highly dynamic during the initiation of the symbiosis and for up to seven days after contact. Three families of bacteria (Haliangiaceae, Pseudoalteromonadacae, Saprospiracae) were shared between the two organisms after symbiosis. Once the symbiosis had been formed, the clownfishes and sea anemone then shared some communities of their mucus microbiota. This study paves the way for further investigations to determine if similar microbial signatures exist in natural environments, whether such microbial sharing can be beneficial for both organisms, and whether the microbiota is implicated in the mechanisms that protect the clownfish from sea anemone stinging.
Conflict of interest statement
The authors declare no competing interests.
Figures
Figure 1
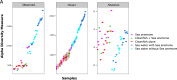
Alpha diversity of the clownfish mucus microbiota (n = 5) in comparison to sea water microbiota (n = 3) with the Chao1 and Shannon indexes respectively showing the richness and phylogenetic diversity of the samples, with sea water being more diverse and richer than the clownfish mucus microbiota.
Figure 2
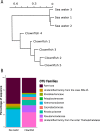
Bacterial diversity and composition of sea water and clownfish mucus samples. (A) Dendrogram showing the specificity of the clownfish mucus compared with the sea water microbiomes. (B) Taxonomic composition, with the percentages of the most representative families of the microbiomes of clownfish mucus and sea water samples.
Figure 3
Alpha diversity of the microbiota of clownfish mucus (n = 15), sea anemone mucus (n = 9) and sea water samples (n = 24), with the Chao1 and Shannon indexes respectively showing the richness and phylogenetic diversity of the samples at each sampling time.
Figure 4
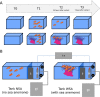
Schematic of the experimental design to monitor the changes in the clownfish and sea anemone mucosal microbiota during the symbiosis, with (A) showing the sampling time with T0 sampling before the introduction of organisms, T1 before contact with the sea anemone, T2 24 hours after the first contact and T3 seven days after the first contact. (B) The experimental aquaria with skimmers (S), heaters (H) controlled by a thermo regulator (TR), brewing pumps (B), and external filters (EF).
Figure 5
Multidimensional scaling analysis showing the changes in the sea water, sea anemone and clownfish mucus microbiota during the symbiosis (A) at T0 prior to the introduction of any organisms in the tanks, (B) at T1 before contact with the sea anemone, (C) at T2 24 hours after the first contact and (D) at T3 seven days after the first contact. Light-coloured points correspond to the sampling time.
Figure 6
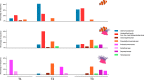
Compositions of sea water and clownfish mucus microbiota (A) at T0 before introduction of any organisms in the tanks, (B) at T1 before contact with the sea anemone, (C) at T2 24 hours after the first contact and (D) at T3 seven days after the first contact. Families between the black lines are those that appeared to be very dynamic in the sampled microbiota.
Figure 7
Composition differences between eight relevant microbiota families in clownfish alone, clownfish with sea anemone and sea anemone before symbiosis (T1) and 24 hours (T2) and seven days after symbiosis (T3).
Similar articles
- Microbiomes of clownfish and their symbiotic host anemone converge before their first physical contact.
Émie AG, François-Étienne S, Sidki B, Nicolas D. Émie AG, et al. Microbiome. 2021 May 17;9(1):109. doi: 10.1186/s40168-021-01058-1. Microbiome. 2021. PMID: 34001275 Free PMC article. - Host identity and symbiotic association affects the taxonomic and functional diversity of the clownfish-hosting sea anemone microbiome.
Titus BM, Laroche R, Rodríguez E, Wirshing H, Meyer CP. Titus BM, et al. Biol Lett. 2020 Feb;16(2):20190738. doi: 10.1098/rsbl.2019.0738. Epub 2020 Feb 5. Biol Lett. 2020. PMID: 32019466 Free PMC article. - Sea anemone-anemonefish symbiosis: Behavior and mucous protein profiling.
Nguyen HT, Zhao M, Wang T, Dang BT, Geffen AJ, Cummins SF. Nguyen HT, et al. J Fish Biol. 2024 Aug;105(2):603-618. doi: 10.1111/jfb.15772. Epub 2024 May 15. J Fish Biol. 2024. PMID: 38747400 - Never, Ever Make an Enemy… Out of an Anemone: Transcriptomic Comparison of Clownfish Hosting Sea Anemone Venoms.
Delgado A, Benedict C, Macrander J, Daly M. Delgado A, et al. Mar Drugs. 2022 Nov 23;20(12):730. doi: 10.3390/md20120730. Mar Drugs. 2022. PMID: 36547877 Free PMC article. Review. - Chemical biology of the mutualistic relationships of sea anemones with fish and crustaceans.
Mebs D. Mebs D. Toxicon. 2009 Dec 15;54(8):1071-4. doi: 10.1016/j.toxicon.2009.02.027. Epub 2009 Mar 5. Toxicon. 2009. PMID: 19268681 Review.
Cited by
- Microbiomes of clownfish and their symbiotic host anemone converge before their first physical contact.
Émie AG, François-Étienne S, Sidki B, Nicolas D. Émie AG, et al. Microbiome. 2021 May 17;9(1):109. doi: 10.1186/s40168-021-01058-1. Microbiome. 2021. PMID: 34001275 Free PMC article. - Anemonefish, a model for Eco-Evo-Devo.
Roux N, Salis P, Lee SH, Besseau L, Laudet V. Roux N, et al. Evodevo. 2020 Oct 7;11:20. doi: 10.1186/s13227-020-00166-7. eCollection 2020. Evodevo. 2020. PMID: 33042514 Free PMC article. Review. - Anemonefishes: A model system for evolutionary genomics.
Herrera M, Ravasi T, Laudet V. Herrera M, et al. F1000Res. 2023 Oct 27;12:204. doi: 10.12688/f1000research.130752.2. eCollection 2023. F1000Res. 2023. PMID: 37928172 Free PMC article. Review.
References
- Barbosa, P. & Castellanos, I. Ecology of Predator-prey Interactions. in Ecology of Predator-prey Interactions (Barbosa, P., Castellanos, I., 2005).
- Fautin DG. The Anemonfish Symbiosis - What is Known and What is Not? Symbiosis. 1991;10(23):46.
- Elliott JK, Elliot JM, Mariscal RN. Host selection, location, and association behaviors of anemonefishes in field settlement experiments. Mar. Biol. 1995;122:377–389. doi: 10.1007/BF00350870. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources