Multidomain ribosomal protein trees and the planctobacterial origin of neomura (eukaryotes, archaebacteria) - PubMed (original) (raw)
Review
Multidomain ribosomal protein trees and the planctobacterial origin of neomura (eukaryotes, archaebacteria)
Thomas Cavalier-Smith et al. Protoplasma. 2020 May.
Abstract
Palaeontologically, eubacteria are > 3× older than neomura (eukaryotes, archaebacteria). Cell biology contrasts ancestral eubacterial murein peptidoglycan walls and derived neomuran N-linked glycoprotein coats/walls. Misinterpreting long stems connecting clade neomura to eubacteria on ribosomal sequence trees (plus misinterpreted protein paralogue trees) obscured this historical pattern. Universal multiprotein ribosomal protein (RP) trees, more accurate than rRNA trees, are taxonomically undersampled. To reduce contradictions with genically richer eukaryote trees and improve eubacterial phylogeny, we constructed site-heterogeneous and maximum-likelihood universal three-domain, two-domain, and single-domain trees for 143 eukaryotes (branching now congruent with 187-protein trees), 60 archaebacteria, and 151 taxonomically representative eubacteria, using 51 and 26 RPs. Site-heterogeneous trees greatly improve eubacterial phylogeny and higher classification, e.g. showing gracilicute monophyly, that many 'rDNA-phyla' belong in Proteobacteria, and reveal robust new phyla Synthermota and Aquithermota. Monoderm Posibacteria and Mollicutes (two separate wall losses) are both polyphyletic: multiple outer membrane losses in Endobacteria occurred separately from Actinobacteria; neither phylum is related to Chloroflexi, the most divergent prokaryotes, which originated photosynthesis (new model proposed). RP trees support an eozoan root for eukaryotes and are consistent with archaebacteria being their sisters and rooted between Filarchaeota (=Proteoarchaeota, including 'Asgardia') and Euryarchaeota sensu-lato (including ultrasimplified 'DPANN' whose long branches often distort trees). Two-domain trees group eukaryotes within Planctobacteria, and archaebacteria with Planctobacteria/Sphingobacteria. Integrated molecular/palaeontological evidence favours negibacterial ancestors for neomura and all life. Unique presence of key pre-neomuran characters favours Planctobacteria only as ancestral to neomura, which apparently arose by coevolutionary repercussions (explained here in detail, including RP replacement) of simultaneous outer membrane and murein loss. Planctobacterial C-1 methanotrophic enzymes are likely ancestral to archaebacterial methanogenesis and β-propeller-α-solenoid proteins to eukaryotic vesicle coats, nuclear-pore-complexes, and intraciliary transport. Planctobacterial chaperone-independent 4/5-protofilament microtubules and MamK actin-ancestors prepared for eukaryote intracellular motility, mitosis, cytokinesis, and phagocytosis. We refute numerous wrong ideas about the universal tree.
Keywords: Archaebacteria; Eubacterial phylogeny; Eukaryogenesis; Ribosomal protein universal tree; Rooting eukaryote trees.
Figures
Fig. 1
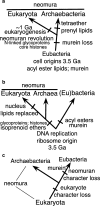
Longstanding contradictory interpretations of the universal rRNA tree. On the ‘eubacteria-first’ view (a), eubacteria are the ancestral domain, several times older than neomura which arose by the neomuran revolution (Cavalier-Smith 1987c, 2002a), a radical cell transformation caused by loss of murein peptidoglycan by a eubacterium similarly to the origins of mycoplasmas and L-forms from Bacillia. a is strongly supported by the fossil record, which indicates that neomura are 3–4 times younger (originating between 0.8 and 1.45 Ga, depending on controversial identification of fossils in this period as ‘stem eukaryotes’ or ‘unusually complex bacteria’: Cavalier-Smith 2006a). Associated changes in cell biology were explained in detail (Cavalier-Smith 2014) on the assumption that the eubacterial ancestor of neomura was a posibacterium (Lake et al. ; Valas and Bourne 2011), whereas new evidence presented here favours the more recent idea that it was a planctobacterium (Reynaud and Devos 2011). It argues that long stems at the base of neomura and eukaryotes on rDNA and RP trees result from episodic hyperacceleration of ribosome evolution caused by origins of cotranslational secretion of glycoproteins and the nucleus respectively (Cavalier-Smith 2002a). The ‘archaea ancient’ view (b) assumes that neomura are as old as eubacteria and that neomuran and eubacterial characters evolved divergently immediately after the origin of life, often assuming that their membranes arose independently by simultaneous separate origins of acyl ester lipids in eubacterial ancestors and isoprenoid ethers in ancestral neomura (this ancient ‘lipid divide’ is now refuted by eubacterial prenyl ether lipids, and archaebacterial fatty acids). b is based on (1) highly dubious a priori ideas about archaebacteria (Woese and Fox 1977a, b); (2) the false assumption that rDNA nucleotide substitution rates have been largely unchanged since cells began; and (3) uncritical interpretation of the first protein paralogue trees that ignored the likelihood that they also are temporally distorted by episodic hyperacceleration causing long-branch artefacts that misroot the three-domain tree in the stretched neomuran stem (Cavalier-Smith 2002a, 2006c). b imagined that eukaryotes replaced isoprenoid ethers by α-proteobacterial acyl esters during mitochondrial enslavement (Martin 1999). Variants of a and b exist that assume that archaebacteria are ancestral to, not sisters of, eukaryotes (Williams et al. 2013), but also accept neomura as a clade. In contrast, the prokaryotes-late or eukaryotes-first (Mariscal and Doolittle 2015) view (c) assumes cells were originally eukaryote-like and prokaryotes arose by radical simplification (‘streamlining’: Forterre 1995) but never explicitly attempted to explain how; Forterre (2013) now prefers b. Proponents of b and c ignore the fossil record that refutes both, and largely ignore cell biology, failing to explain how assumed cell transformations could have occurred (incredible for c; highly implausible selectively and mechanistically for b—yet b may still be the most widespread assumption despite its serious defects; many remain unaware that paralogue pairs more often favour a eubacterial root, like fossils). Only a offers a scientifically explicit hypothesis as to the cell structure of LUCA
Fig. 2
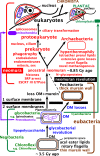
The major kinds of cell and likely evolutionary relationships. Cell envelope and chromosome chemistry divides life into ancestral eubacteria, with murein peptidoglycan walls and DNA negatively supercoiled by DNA gyrase without histones, and derived neomura (probably over three times younger), with N-glycoproteins cotranslationally secreted by more complex SRPs and DNA passively negatively supercoiled by histones (some archaebacteria may retain eubacterial DNA gyrase and reverse gyrase and some lost histones). Eubacteria exhibit three grades of organisation: Chloroflexi (=Chlorobacteria), unusual negibacteria with an outer membrane (OM) of phospholipids but no lipopolysaccharide (LPS); glycobacteria, the majority of negibacteria (11 phyla), whose OM has an outer leaflet of LPS: and monoderm posibacteria whose ancestors lost the OM and comprise a majority of phyla Actinobacteria and Endobacteria. We argue that neomura arose after simultaneous loss of murein and OM by a planctobacterial glycobacterium with primitive microtubules; numerous recent discoveries make the older idea based on OM loss parsimony (Cavalier-Smith 1987c) that they arose from a posibacterium by losing murein only (dashed line) no longer tenable. Eukaryotes kept eubacterial acyl ester lipids but archaebacteria became hyperthermophiles by largely replacing them by stabler prenyl ether lipids (whose biosynthetic enzymes and diether variants probably arose much earlier in glycobacteria). Archaebacteria retained prokaryote cell structure and DNA segregation machinery but not microtubules, whereas eukaryotes evolved phagotrophy that caused evolution of an endomembrane system with coated vesicle budding and targeted vesicle fusion leading to origin of the nucleus, microtubule-based mitosis and consequential radical genetic changes, and enabled intracellular symbiogenesis by enslaving glycobacteria: a chromatophore-bearing α-proteobacterium as mitochondria to make kingdom Protozoa; and later a thylakoid-bearing cyanobacterium as chloroplasts to make kingdom Plantae. Kingdoms Eubacteria and Archaebacteria have non-homologous rotary extracellular flagella; but eukaryotes all descend from an ancestral biciliate protozoan with two immensely more complex microtubule-based intracellular bending cilia that undergo structural transformation once every cell cycle, the younger one losing its juvenile morphology in the second cell cycle. We do not portray the most complex membrane topology of all, found in kingdom Chromista, where chloroplasts, a red algal plasma membrane, and sometimes a relict nucleus, are present inside host ER lumen, having arisen soon after chloroplasts when a biciliate phagotroph enslaved an engulfed red algal symbiont (see Cavalier-Smith 2018). Eukaryogenesis is postulated to have involved three logically distinct stages (asterisks); mitochondria must have preceded spliceosomes and followed the prekaryote phase but might have become symbionts simultaneously with nucleus and cilium origins
Fig. 3
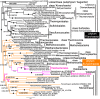
Site-heterogeneous PhyloBayes CAT-GTR tree for 51 ribosomal proteins from 143 eukaryotes representing all the most divergent lineages. Support values for bipartitions are from left to right: posterior probabilities for CAT-GTR, posterior probabilities for CAT-Poisson, RAxML bootstrap percentages for 100 pseudoreplicates; black blobs signify maximal support by all methods in this and all other figures. To fit the page branches for major taxa are collapsed and the number of species included in each given beside their label; their names are shown on uncollapsed trees in Supplementary material, e.g. Fig. S1. The CAT-GTR tree summed 103,304 trees after removing 40% as burn in; both chains converged satisfactorily - maxdiff 0.276977. The CAT-Poisson tree summed 201,391 trees after removing 20% as burn in, but its two chains had slightly different topology (see text)—maxdiff 0.96. The tree is rooted within Eozoa between discicristates and jakobids, but leaving their relative branching order compared with Tsukubamonas as an unresolved trifurcation as it is unclear whether Tsukubamonas is more closely related to jakobids or to discicristates or the deepest branching lineage (see Cavalier-Smith 2017, 2018). However, it remains controversial whether Eozoa is the basal eukaryote group as shown or whether it is a clade (see text and Fig. 6); in any case, the bifurcation between Eozoa and neokaryotes is the most strongly supported dichotomy on the basal backbone of the RP tree. Compared with Eozoa, whose deep branches are well spread out and fully resolved by all methods, basal branches of neokaryotes form an explosively rapid radiation that is necessarily relatively poorly resolved
Fig. 4
Site-heterogeneous PhyloBayes CAT-GTR tree for 51 ribosomal proteins from 60 archaebacteria representing all the most divergent lineages. Support values for bipartitions are from left to right: posterior probabilities for the CAT-GTR chain 1 analysis (50,437 trees summed after removing 40% of trees as burnin; chain 2 was identical except for rhe position of ‘Nanohaloarchaea’ which were sister to Aenigmarchaeota/GWA2_AR5 in the position shown by arrow NH2 as also on the ML tree), posterior probabilities for the CAT-Poisson (126,435 trees summed after removing 20% as burnin: maxdiff 1), RAxML bootstrap percentages for 100 pseudoreplicates. Arrow NHP shows the contradictory position of Nanohaloarchaea on CAT-Poisson analyses. Asgard archaebacteria are represented only by lokiarchaeotes as other sublineages were unavailable when our analyses began
Fig. 5
Site-heterogeneous PhyloBayes CAT-GTR tree for 26 ribosomal proteins from 151 eubacteria representing all the most divergent lineages with cultivated representatives plus Melainabacteria and chloroplasts. Support values for bipartitions are from left to right: posterior probabilities for the CAT-GTR, posterior probabilities (PP) for the CAT-Poisson, RAxML bootstrap percentages for 100 pseudoreplicates. To fit on the page branches for some major taxa are collapsed and the numbers of species included for each given beside their label; their names are shown on uncollapsed trees in Supplementary material, e.g. Figs. S9, S10. Despite 70,629 trees being summed after removing the first 30% of them as burnin the two chains did not converge (maxdiff 1) because of two persistent topological differences within Endobacteria at nodes where PP are shown in red. The CAT-Poisson tree did converge (maxdiff 0.328 after we removed 20% as burnin and summed 89,031 trees) on a slightly different topology that also implies five OM losses within Endobacteria; all 14 phyla were clades; branching order of phyla was the same except that Hadobacteria and Fusobacteria were sisters (0.6 support) and Sphingobacteria sisters (0.72) to Spirochaetes not Planctobacteria. The six probably ancestrally monoderm clades are marked by an open brown oval. All others were ancestrally negibacteria with a porin-bearing OM (the two in Endobacteria are labelled OM). Polyphyletic wall-less mollicutes are marked by a black blob beside their names
Fig. 6.
Site-heterogeneous PhyloBayes CAT-GTR tree for 51 ribosomal proteins from 203 neomura representing all the most divergent lineages. Support values for bipartitions are: posterior probabilities for CAT-GTR (left; 51,189 trees summed after removing 40% as burnin: maxdiff 1; convergence was prevented by four persisting contradictions deeply within neokaryotes), RAxML bootstrap percentages for 100 pseudoreplicates (right). To fit the page branches for major taxa are collapsed; all names are shown on uncollapsed trees in Supplementary material, e.g. Fig. S1. Includes all taxa from Figs. 3 and 4
Fig. 7
Site-heterogeneous prokaryote PhyloBayes CAT-GTR tree for 26 ribosomal proteins from 60 archaebacteria and 151 eubacteria representing all the most divergent lineages. Consensus of two chains; support values for bipartitions are posterior probabilities for the CAT-GTR (left; after removing 40% as burnin 179,537 trees summed; maxdiff 0.179537), RAxML bootstrap percentages for 100 pseudoreplicates (right). To fit on the page branches for major taxa are collapsed; their names are on uncollapsed trees in Supplementary material, e.g. Fig. S9. Archaebacteria are strongly excluded from Posibacteria and branch within Neonegibacteria. weakly as sister to Planctochora
Fig. 8
Site-heterogeneous 2-domain PhyloBayes CAT-GTR tree for 51 ribosomal proteins from 143 eukaryotes and 26 ribosomal proteins from 151 eubacteria representing all the most divergent lineages. Support values for bipartitions are from left to right: posterior probabilities for the CAT-GTR (left), RAxML bootstrap percentages for 100 pseudoreplicates (right). To fit on the page branches for major taxa are collapsed; their names are shown on uncollapsed trees in Supplementary material, e.g. Fig. S12. Despite 33,393 trees being summed after removing the first 17,893 as burnin the two chains did not converge (maxdiff 1) because of a few persistent topological differences (with 0.5 support or less) at the base of neonegibacteria and neokaryotes; both strongly excluded eukaryotes from Posibacteria and placed them within gracilicute Neonegibacteria. The root of eukaryotes beside Percolozoa within Eozoa was the same on both chains; one chain placed eukaryotes within Planctobacteria as on the consensus tree, but more strongly so, whereas the other put them more weakly two nodes more deeply as sister to Planctochlora
Fig. 9
Site-heterogeneous universal three-domain PhyloBayes CAT-GTR tree for 26 ribosomal proteins from 143 eukaryotes, 60 archaebacteria, and 151 eubacteria representing all the most divergent lineages. Support values for bipartitions are from left to right: posterior probabilities for the CAT-GTR (left), RAxML bootstrap percentages for 100 pseudoreplicates (right). To fit on the page, branches for major taxa are collapsed; their names are shown on uncollapsed trees in Supplementary material, e.g. Fig. S1. As the chains did not converge, this figure is for chain 2 with ML support values also mapped on to it. After removing the first 20% as burnin, the remaining 19,165 trees were summed. Deep branching order of prokaryote phyla is markedly more disturbed than in 2-domain trees (Figs. 6, 7, and 8)
Fig. 10
Site-heterogeneous universal three-domain PhyloBayes CAT-GTR tree for 51 ribosomal proteins from 143 eukaryotes and 60 archaebacteria and 26 ribosomal proteins from 151 eubacteria representing all the most divergent lineages. Support values for bipartitions are from left to right: posterior probabilities for the CAT-GTR (84.962 trees summed from four independent chains after removing 4035 trees as burnin: maxdiff 1), posterior probabilities for the CAT-Poisson (29,287 trees summed after removing 9,872 treees as burnin), RAxML bootstrap percentages for 100 pseudoreplicates. To fit on the page branches for major taxa are collapsed; their names are shown on uncollapsed trees in Supplementary material, e.g. Fig. S1
Fig. 11
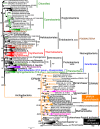
The 14 eubacterial phyla recognised here. For two exceptionally diverse phyla (Proteobacteria, Sphingobacteria) their three major subbranches, here ranked as subphyla (but often treated as several smaller phyla), are also shown. Support for the monophyly of each (from Fig. 5) is extremely high as is CAT-tree support from Fig. 5 for their relative branching order, except for the position of Hadobacteria which sometimes appear as sister to Fusobacteria (dashed arrow). Their branching order is otherwise very stable on site-heterogeneous trees restricted to Eubacteria, but adding one or both highly divergent neomuran groups on multidomain trees makes branching order less stable, there being a strong tendency for the major thermophilic phyla (Aquithermota, Synthermota) to group together or become partially intermixed with Hadobacteria/Fusobacteria; these changes are likely artefacts. Phyla with some photosynthetic members are in green; the different types of photosynthetic reaction centres (RC and characteristic deletions) and presence of FMO, chlorins, phycobilisomes (PB) and chlorosomes (cs) are mapped onto the tree; it is unknown if uncultured Candidatus Palusbacteriales (‘Eremiobacteria’: Ward et al. 2019) has chlorosomes—as not in our analyses, its likely position in Armatimonadetes (dashed line) is only weakly established; its discovery increases the likelihood that ancestral eubacteria (i.e. LUCA) had RCII. The position of neomura (dashed line) is based on two-domain RP trees (see text)
Fig. 12
SMC site-heterogeneous PhyloBayes CAT-GTR phylogeny for 305 prokaryotes using 448 amino acid positions. Two chains were run and summed; after convergence (maxdiff. 0.209461) 40% of trees were removed as burnin. To fit on the page many clades were collapsed (numbers of species in each noted on the right); all names are on the corresponding uncollapsed tree (supplementary Fig. S17). Bipartition support values are posterior probabilities
Fig. 13
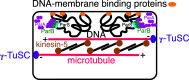
Origin of premitosis is crucial for eukaryogenesis. It is argued that prokaryotic ParABS-based DNA segregation and MinD-based cell polarity must have been retained for cell viability during eukaryogenesis when origin of centromeric nucleation of 13-pf mts by γ-TuSC originated a novel cell polarity mechanism, and origin of bipolar kinesin-5 enabled centrosome-nucleated mts to form an intercentrosomal mitotic spindle able to mediate anaphase B. Only then could anaphase A (not shown) evolve by conversion and/or replacement of ParB to/by inner kinetochore proteins and their slideable linkage by outer kinetochore Ndc80 rods to mt + ends depolymerisable by novel kinesin-8, as the text summarises. This sequence of events leaves no period during eukaryogenesis without a workable DNA segregation machinery and explains simply how both prokaryotic segregation and cell polarity mechanisms based on protein diffusion ratchets could be harmlessly replaced by radically different eukaryotic mt/kinesin motor mechanisms. Once centromeric mts took over the general polarity function of bacterial MinD they provided the structural framework on which other kinesin mt motors, then dyneins, could act and also stable polarity on a larger spatial scale than can prokaryotes by Min, MreB, and FtsZ, and thus help organise the now branched actin network
Similar articles
- The neomuran origin of archaebacteria, the negibacterial root of the universal tree and bacterial megaclassification.
Cavalier-Smith T. Cavalier-Smith T. Int J Syst Evol Microbiol. 2002 Jan;52(Pt 1):7-76. doi: 10.1099/00207713-52-1-7. Int J Syst Evol Microbiol. 2002. PMID: 11837318 Review. - Rooting the tree of life by transition analyses.
Cavalier-Smith T. Cavalier-Smith T. Biol Direct. 2006 Jul 11;1:19. doi: 10.1186/1745-6150-1-19. Biol Direct. 2006. PMID: 16834776 Free PMC article. - The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa.
Cavalier-Smith T. Cavalier-Smith T. Int J Syst Evol Microbiol. 2002 Mar;52(Pt 2):297-354. doi: 10.1099/00207713-52-2-297. Int J Syst Evol Microbiol. 2002. PMID: 11931142 Review. - The neomuran revolution and phagotrophic origin of eukaryotes and cilia in the light of intracellular coevolution and a revised tree of life.
Cavalier-Smith T. Cavalier-Smith T. Cold Spring Harb Perspect Biol. 2014 Sep 2;6(9):a016006. doi: 10.1101/cshperspect.a016006. Cold Spring Harb Perspect Biol. 2014. PMID: 25183828 Free PMC article. Review. - Predation and eukaryote cell origins: a coevolutionary perspective.
Cavalier-Smith T. Cavalier-Smith T. Int J Biochem Cell Biol. 2009 Feb;41(2):307-22. doi: 10.1016/j.biocel.2008.10.002. Epub 2008 Oct 18. Int J Biochem Cell Biol. 2009. PMID: 18935970 Review.
Cited by
- The soil microbial food web revisited: Predatory myxobacteria as keystone taxa?
Petters S, Groß V, Söllinger A, Pichler M, Reinhard A, Bengtsson MM, Urich T. Petters S, et al. ISME J. 2021 Sep;15(9):2665-2675. doi: 10.1038/s41396-021-00958-2. Epub 2021 Mar 21. ISME J. 2021. PMID: 33746204 Free PMC article. - Agl24 is an ancient archaeal homolog of the eukaryotic N-glycan chitobiose synthesis enzymes.
Meyer BH, Adam PS, Wagstaff BA, Kolyfetis GE, Probst AJ, Albers SV, Dorfmueller HC. Meyer BH, et al. Elife. 2022 Apr 8;11:e67448. doi: 10.7554/eLife.67448. Elife. 2022. PMID: 35394422 Free PMC article. - The Evolution of the Hallmarks of Aging.
Lemoine M. Lemoine M. Front Genet. 2021 Aug 26;12:693071. doi: 10.3389/fgene.2021.693071. eCollection 2021. Front Genet. 2021. PMID: 34512720 Free PMC article. Review. - Phylogenetic analysis of mutational robustness based on codon usage supports that the standard genetic code does not prefer extreme environments.
Radványi Á, Kun Á. Radványi Á, et al. Sci Rep. 2021 May 26;11(1):10963. doi: 10.1038/s41598-021-90440-y. Sci Rep. 2021. PMID: 34040064 Free PMC article. - Evolutionary Origins of DNA Repair Pathways: Role of Oxygen Catastrophe in the Emergence of DNA Glycosylases.
Prorok P, Grin IR, Matkarimov BT, Ishchenko AA, Laval J, Zharkov DO, Saparbaev M. Prorok P, et al. Cells. 2021 Jun 24;10(7):1591. doi: 10.3390/cells10071591. Cells. 2021. PMID: 34202661 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources