Docetaxel-Loaded Disulfide Cross-Linked Nanoparticles Derived from Thiolated Sodium Alginate for Colon Cancer Drug Delivery - PubMed (original) (raw)
Docetaxel-Loaded Disulfide Cross-Linked Nanoparticles Derived from Thiolated Sodium Alginate for Colon Cancer Drug Delivery
Hock Ing Chiu et al. Pharmaceutics. 2020.
Abstract
In this study, fluorescein-labelled wheat germ agglutinin (fWGA)-conjugated disulfide cross-linked sodium alginate nanoparticles were developed to specifically target docetaxel (DTX) to colon cancer cells. Different amounts of 3-mercaptopropionic acid (MPA) were covalently attached to sodium alginate to form thiolated sodium alginate (MPA1-5). These polymers were then self-assembled and air-oxidised to form disulfide cross-linked nanoparticles (MP1-5) under sonication. DTX was successfully loaded into the resulting MP1-5 to form DTX-loaded nanoparticles (DMP1-5). DMP2 had the highest loading efficiency (17.8%), thus was chosen for fWGA surface conjugation to form fWGA-conjugated nanoparticles (fDMP2) with a conjugation efficiency of 14.1%. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) analyses showed spherical nanoparticles, and an in vitro drug release study recorded a cumulative drug release of 48.6%. Dynamic light scattering (DLS) analysis revealed a mean diameter (MD) of 289 nm with a polydispersity index (PDI) of 0.3 and a zeta potential of -2.2 mV for fDMP2. HT-29 human colon cancer cells treated with fDMP2 showed lower viability than that of L929 mouse fibroblast cells. These results indicate that fDMP2 was efficiently taken up by HT-29 cells (29.9%). Fluorescence and confocal imaging analyses also showed possible internalisation of nanoparticles by HT-29 cells. In conclusion, fDMP2 shows promise as a DTX carrier for colon cancer drug delivery.
Keywords: HT-29; disulfide cross-linked nanoparticles; docetaxel; thiolated sodium alginate; wheat germ agglutinin conjugation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
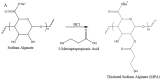
Scheme 1
Synthetic scheme of (A) MPA and (B) assembly process of disulfide cross-linked nanoparticles.
Scheme 1
Synthetic scheme of (A) MPA and (B) assembly process of disulfide cross-linked nanoparticles.
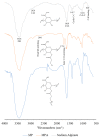
Figure 1
FTIR spectra of sodium alginate, MPA and MP.
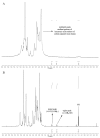
Figure 2
1H-NMR spectra of (A) sodium alginate and (B) MPA.
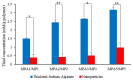
Figure 3
Comparison of the concentrations of thiol between MPA polymers and MP nanoparticles (mean ± SD, n = 3); ** (p < 0.01) and * (p < 0.05) indicate significant differences between the samples.
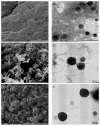
Figure 4
SEM micrographs of (A) MP2, (C) DMP2, (E) fDMP2; and TEM micrographs of (B) MP2, (D) DMP2, (F) fDMP2.
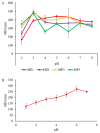
Figure 5
pH sensitivity profiles of (A) MP1–5 and (B) fMP2 (mean ± SD, n = 3).
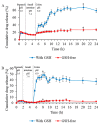
Figure 6
In vitro drug release profile of (A) DMP2 and (B) fDMP2 in control media (GSH-free) and in stimulated gastrointestinal media (with GSH) at predetermined pH and time (mean ± SD, n = 3); * (p < 0.05) indicates that significant differences were observed between the samples.
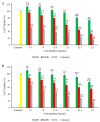
Figure 7
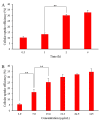
Comparison of effects of fMP2, fDMP2, and DTX on (A) HT-29 cells and (B) L929 cells (mean ± SD, n = 3); the samples with same alphabets a–r indicate significant difference between them, while, sample with * indicates (p < 0.05) compared to untreated cells.
Figure 8
Cellular uptake profile of fDMP2 by HT-29 cells as a function of (A) incubation time and (B) concentration over an incubation period of 2 h (mean ± SD, n = 3); ** indicates p < 0.01 between the two time intervals.
Figure 9
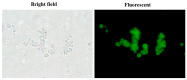
Cellular uptake of fDMP2 by HT-29 cells (2 h) under 40,000 × magnifications illustrated by fluorescence microscopy.
Figure 10
Colocalisation of clathrin for fDMP2 in HT-29 cells; localisation of clathrin, nucleus, nanoparticles, and the merged image in HT-29 cells.
Similar articles
- Wheat Germ Agglutinin-Conjugated Disulfide Cross-Linked Alginate Nanoparticles as a Docetaxel Carrier for Colon Cancer Therapy.
Chiu HI, Lim V. Chiu HI, et al. Int J Nanomedicine. 2021 Apr 22;16:2995-3020. doi: 10.2147/IJN.S302238. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33911862 Free PMC article. - Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management.
Kushwah V, Katiyar SS, Dora CP, Kumar Agrawal A, Lamprou DA, Gupta RC, Jain S. Kushwah V, et al. Acta Biomater. 2018 Jun;73:424-436. doi: 10.1016/j.actbio.2018.03.057. Epub 2018 Apr 9. Acta Biomater. 2018. PMID: 29649635 - Preparation, characterization, and in vitro drug release behavior of thiolated alginate nanoparticles loaded budesonide as a potential drug delivery system toward inflammatory bowel diseases.
Arif M, Chi Z, Liu YJ, Liu CG. Arif M, et al. J Biomater Sci Polym Ed. 2020 Dec;31(18):2299-2317. doi: 10.1080/09205063.2020.1803034. Epub 2020 Sep 3. J Biomater Sci Polym Ed. 2020. PMID: 32727293 - Biocompatible disulphide cross-linked sodium alginate derivative nanoparticles for oral colon-targeted drug delivery.
Ayub AD, Chiu HI, Mat Yusuf SNA, Abd Kadir E, Ngalim SH, Lim V. Ayub AD, et al. Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):353-369. doi: 10.1080/21691401.2018.1557672. Artif Cells Nanomed Biotechnol. 2019. PMID: 30691309 - Preparation, characterization, and in vitro evaluation of docetaxel-loaded poly(lactic acid)-poly(ethylene glycol) nanoparticles for parenteral drug delivery.
Liu D, Wang L, Liu Z, Zhang C, Zhang N. Liu D, et al. J Biomed Nanotechnol. 2010 Dec;6(6):675-82. doi: 10.1166/jbn.2010.1160. J Biomed Nanotechnol. 2010. PMID: 21361132
Cited by
- K. ZHENG ET AL.Gold-nanoparticle-based multistage drug delivery system for antitumor therapy.
Zheng K, Zhou D, Wu L, Li J, Zhao B, Zhang S, He R, Xiao L, Zoya I, Yu L, Zhang Y, Li Y, Gao J, Li K. Zheng K, et al. Drug Deliv. 2022 Dec;29(1):3186-3196. doi: 10.1080/10717544.2022.2128469. Drug Deliv. 2022. PMID: 36226475 Free PMC article. - Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment.
Wong KH, Lu A, Chen X, Yang Z. Wong KH, et al. Molecules. 2020 Aug 9;25(16):3620. doi: 10.3390/molecules25163620. Molecules. 2020. PMID: 32784890 Free PMC article. Review. - Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art.
Abourehab MAS, Rajendran RR, Singh A, Pramanik S, Shrivastav P, Ansari MJ, Manne R, Amaral LS, Deepak A. Abourehab MAS, et al. Int J Mol Sci. 2022 Aug 12;23(16):9035. doi: 10.3390/ijms23169035. Int J Mol Sci. 2022. PMID: 36012297 Free PMC article. Review. - Targeting the Gut: A Systematic Review of Specific Drug Nanocarriers.
Garbati P, Picco C, Magrassi R, Signorello P, Cacopardo L, Dalla Serra M, Faticato MG, De Luca M, Balestra F, Scavo MP, Viti F. Garbati P, et al. Pharmaceutics. 2024 Mar 21;16(3):431. doi: 10.3390/pharmaceutics16030431. Pharmaceutics. 2024. PMID: 38543324 Free PMC article. Review. - 2-Hydroxyoleic Acid as a Self-Assembly Inducer for Anti-Cancer Drug-Centered Nanoparticles.
Antoniou AI, Nordio G, Di Paolo ML, Colombo E, Gaffuri B, Polito L, Amenta A, Seneci P, Dalla Via L, Perdicchia D, Passarella D. Antoniou AI, et al. Pharmaceuticals (Basel). 2023 May 9;16(5):722. doi: 10.3390/ph16050722. Pharmaceuticals (Basel). 2023. PMID: 37242505 Free PMC article.
References
- Azizah A.M., Nor Saleha I.T., Noor Hashimah A., Asmah Z.A., Mastulu W. Malaysian National Cancer Registry Report 2007-2011. National Cancer Institute, Ministry of Health; Putrajaya, Malaysia: 2016. pp. 29–38.
LinkOut - more resources
Full Text Sources