Randomized, Controlled Trial of Tacrolimus and Prednisolone Monotherapy for Adults with De Novo Minimal Change Disease: A Multicenter, Randomized, Controlled Trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2020 Feb 7;15(2):209-218.
doi: 10.2215/CJN.06180519. Epub 2020 Jan 17.
Christopher Lawrence 2, Marie Condon 3, Bhrigu Sood 3, Paul Warwicker 2, Heather Brown 4, James Pattison 4, Sunil Bhandari 5, Jonathan Barratt 6, Neil Turner 7, H Terence Cook 1 8, Jeremy B Levy 1, Liz Lightstone 1 8, Charles Pusey 1 8, Jack Galliford 1, Thomas D Cairns 1, Megan Griffith 1
Affiliations
- PMID: 31953303
- PMCID: PMC7015084
- DOI: 10.2215/CJN.06180519
Randomized Controlled Trial
Randomized, Controlled Trial of Tacrolimus and Prednisolone Monotherapy for Adults with De Novo Minimal Change Disease: A Multicenter, Randomized, Controlled Trial
Nicholas Rhys Medjeral-Thomas et al. Clin J Am Soc Nephrol. 2020.
Erratum in
- Correction.
[No authors listed] [No authors listed] Clin J Am Soc Nephrol. 2020 Jul 1;15(7):1027. doi: 10.2215/CJN.06290420. Epub 2020 Jun 9. Clin J Am Soc Nephrol. 2020. PMID: 32518101 Free PMC article. No abstract available.
Abstract
Background and objectives: Minimal change disease is an important cause of nephrotic syndrome in adults. Corticosteroids are first-line therapy for minimal change disease, but a prolonged course of treatment is often required and relapse rates are high. Patients with minimal change disease are therefore often exposed to high cumulative corticosteroid doses and are at risk of associated adverse effects. This study investigated whether tacrolimus monotherapy without corticosteroids would be effective for the treatment of de novo minimal change disease.
Design, setting, participants, & measurements: This was a multicenter, prospective, open-label, randomized, controlled trial involving six nephrology units across the United Kingdom. Adult patients with first presentation of minimal change disease and nephrotic syndrome were randomized to treatment with either oral tacrolimus at 0.05 mg/kg twice daily, or prednisolone at 1 mg/kg daily up to 60 mg daily. The primary outcome was complete remission of nephrotic syndrome after 8 weeks of therapy. Secondary outcomes included remission of nephrotic syndrome at 16 and 26 weeks, rates of relapse of nephrotic syndrome, and changes from baseline kidney function.
Results: There were no significant differences between the tacrolimus and prednisolone treatment cohorts in the proportion of patients in complete remission at 8 weeks (21 out of 25 [84%] for prednisolone and 17 out of 25 [68%] for tacrolimus cohorts; _P_=0.32; difference in remission rates was 16%; 95% confidence interval [95% CI], -11% to 40%), 16 weeks (23 out of 25 [92%] for prednisolone and 19 out of 25 [76%] for tacrolimus cohorts; _P_=0.25; difference in remission rates was 16%; 95% CI, -8% to 38%), or 26 weeks (23 out of 25 [92%] for prednisolone and 22 out of 25 [88%] for tacrolimus cohorts; _P_=0.99; difference in remission rates was 4%; 95% CI, -17% to 25%). There was no significant difference in relapse rates (17 out of 23 [74%] for prednisolone and 16 out of 22 [73%] for tacrolimus cohorts) for patients in each group who achieved complete remission _(P_=0.99) or in the time from complete remission to relapse.
Conclusions: Tacrolimus monotherapy can be effective alternative treatment for patients wishing to avoid steroid therapy for minimal change disease.
Podcast: This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2020\_01\_16\_CJN06180519.mp3.
Keywords: Glomerulonephritis; United Kingdom; adult; humans; lipoid nephrosis; nephrology; nephrotic syndrome; prednisolone; prospective studies; recurrence; remission induction; tacrolimus; treatment outcome.
Copyright © 2020 by the American Society of Nephrology.
Figures
Graphical abstract
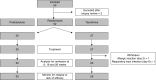
Figure 1.
Study design and study cohort flowchart. Of 55 patients enrolled, 52 were randomized to treatment, and 25 patients in each cohort completed treatment. Two patients in the tacrolimus group did not complete treatment; one patient developed diarrhea and rash 2 days after starting tacrolimus; one patient developed lower respiratory tract infection 8 days into treatment; both were withdrawn from the study to receive nonprotocol treatment. These patients are included in the intention-to-treat analysis and excluded from the per-protocol analysis.
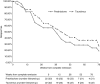
Figure 2.
The proportion of patients achieving complete remission. (A) Complete remission and (B) all remission (complete or partial remission) against weeks from the start of treatment in the prednisolone (gray, solid line) and tacrolimus (black, dashed line) cohorts. The number of patients achieving remission is shown in the table below each graph.
Figure 3.
The proportion of patients who suffered a relapse after complete remission in the prednisolone (gray, solid line) and tacrolimus (black, dashed line) cohorts. The number of patients with relapse after complete remission at 26-week intervals is shown. Two of the patients in remission in the prednisolone group were lost to follow-up at 36 weeks and 40 weeks.
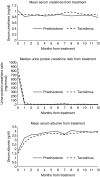
Figure 4.
Changes in mean serum creatinine, median urine protein-to-creatinine ratio, and mean serum albumin from the start of treatment and during follow-up in the prednisolone and tacrolimus treated cohorts. The urine protein-to-creatinine ratio data were nonparametrically distributed.
Similar articles
- Comparison of the Efficacy and Safety of Tacrolimus and Low-Dose Corticosteroid with High-Dose Corticosteroid for Minimal Change Nephrotic Syndrome in Adults.
Chin HJ, Chae DW, Kim YC, An WS, Ihm C, Jin DC, Kim SG, Kim YL, Kim YS, Kim YG, Koo HS, Lee JE, Lee KW, Oh J, Park JH, Jiang H, Lee H, Lee SK. Chin HJ, et al. J Am Soc Nephrol. 2021 Jan;32(1):199-210. doi: 10.1681/ASN.2019050546. Epub 2020 Nov 9. J Am Soc Nephrol. 2021. PMID: 33168602 Free PMC article. Clinical Trial. - Treatment with tacrolimus and prednisolone is preferable to intravenous cyclophosphamide as the initial therapy for children with steroid-resistant nephrotic syndrome.
Gulati A, Sinha A, Gupta A, Kanitkar M, Sreenivas V, Sharma J, Mantan M, Agarwal I, Dinda AK, Hari P, Bagga A. Gulati A, et al. Kidney Int. 2012 Nov;82(10):1130-5. doi: 10.1038/ki.2012.238. Epub 2012 Jul 4. Kidney Int. 2012. PMID: 22763815 Clinical Trial. - Mycophenolate mofetil is inferior to tacrolimus in sustaining remission in children with idiopathic steroid-resistant nephrotic syndrome.
Sinha A, Gupta A, Kalaivani M, Hari P, Dinda AK, Bagga A. Sinha A, et al. Kidney Int. 2017 Jul;92(1):248-257. doi: 10.1016/j.kint.2017.01.019. Epub 2017 Mar 18. Kidney Int. 2017. PMID: 28318625 Clinical Trial. - Interventions for minimal change disease in adults with nephrotic syndrome.
Palmer SC, Nand K, Strippoli GF. Palmer SC, et al. Cochrane Database Syst Rev. 2008 Jan 23;2008(1):CD001537. doi: 10.1002/14651858.CD001537.pub4. Cochrane Database Syst Rev. 2008. PMID: 18253993 Free PMC article. Updated. Review. - Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome.
Chen Y, Schieppati A, Chen X, Cai G, Zamora J, Giuliano GA, Braun N, Perna A. Chen Y, et al. Cochrane Database Syst Rev. 2014 Oct 16;2014(10):CD004293. doi: 10.1002/14651858.CD004293.pub3. Cochrane Database Syst Rev. 2014. PMID: 25318831 Free PMC article. Updated. Review.
Cited by
- An Updated Comprehensive Review on Diseases Associated with Nephrotic Syndromes.
Wendt R, Sobhani A, Diefenhardt P, Trappe M, Völker LA. Wendt R, et al. Biomedicines. 2024 Oct 4;12(10):2259. doi: 10.3390/biomedicines12102259. Biomedicines. 2024. PMID: 39457572 Free PMC article. Review. - Anti-Tumor Necrosis Factor Treatment for Glomerulopathy: Case Report and Review of Literature.
Jones OY, Malone LC, Brunson C. Jones OY, et al. Fed Pract. 2024 Aug;41(8):250-255. doi: 10.12788/fp.0506. Epub 2024 Aug 15. Fed Pract. 2024. PMID: 39410922 Free PMC article. - Management of immune-mediated glomerular diseases in the elderly.
Angioi A, Cheungpasitporn W, Lepori N. Angioi A, et al. Ren Fail. 2024 Dec;46(2):2411848. doi: 10.1080/0886022X.2024.2411848. Epub 2024 Oct 8. Ren Fail. 2024. PMID: 39378123 Free PMC article. Review. - A randomised, two-arm (1:1 ratio), double blind, placebo controlled phase III trial to assess the efficacy, safety, cost and cost-effectiveness of Rituximab in treating de novo or relapsing NS in patients with MCD/FSGS (TURING).
C Willcocks L, Qian W, Cader R, Gatley K, Siddiqui H, Tabebisong E, Champion K, Kronbichler A, Lightstone L, Jayne D, Wilson E, Griffith M. C Willcocks L, et al. BMC Nephrol. 2024 Aug 7;25(1):253. doi: 10.1186/s12882-024-03576-0. BMC Nephrol. 2024. PMID: 39112932 Free PMC article. Clinical Trial. - Drug repurposing for glomerular diseases: an underutilized resource.
Ng MSY, Kaur G, Francis RS, Hawley CM, Johnson DW. Ng MSY, et al. Nat Rev Nephrol. 2024 Nov;20(11):707-721. doi: 10.1038/s41581-024-00864-8. Epub 2024 Jul 31. Nat Rev Nephrol. 2024. PMID: 39085415 Review.
References
- Haas M, Meehan SM, Karrison TG, Spargo BH: Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis 30: 621–631, 1997. - PubMed
- Gesualdo L, Di Palma AM, Morrone LF, Strippoli GF, Schena FP; Italian Immunopathology Group, Italian Society of Nephrology : The Italian experience of the national registry of renal biopsies. Kidney Int 66: 890–894, 2004. - PubMed
- Tune BM, Mendoza SA: Treatment of the idiopathic nephrotic syndrome: Regimens and outcomes in children and adults. J Am Soc Nephrol 8: 824–832, 1997. - PubMed
- Huang JJ, Hsu SC, Chen FF, Sung JM, Tseng CC, Wang MC: Adult-onset minimal change disease among Taiwanese: Clinical features, therapeutic response, and prognosis. Am J Nephrol 21: 28–34, 2001. - PubMed
- McIntyre P, Craig JC: Prevention of serious bacterial infection in children with nephrotic syndrome. J Paediatr Child Health 34: 314–317, 1998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical