Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2020 Jun;22(6):929-937.
doi: 10.1111/dom.13978. Epub 2020 Feb 14.
Affiliations
- PMID: 31984646
- PMCID: PMC7317838
- DOI: 10.1111/dom.13978
Randomized Controlled Trial
Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial
Lawrence Blonde et al. Diabetes Obes Metab. 2020 Jun.
Abstract
Aim: To compare the effect of liraglutide or placebo added on to sodium-glucose co-transporter-2 inhibitor (SGLT2i) ± metformin on glycaemic control in patients with type 2 diabetes.
Materials and methods: Patients with type 2 diabetes on a stable SGLT2i dose ± metformin (with HbA1c 7.0%-9.5% and body mass index [BMI] ≥ 20 kg/m2 ) were randomized 2:1 to add-on liraglutide 1.8 mg/day or placebo in this parallel, double-blind, multinational trial. Primary and confirmatory secondary endpoints were changes in HbA1c and body weight from baseline to week 26, respectively. The proportions of patients achieving HbA1c (<7.0%) targets and safety events after week 26 were also assessed.
Results: Of 303 patients randomized (one in error), 280 completed treatment. Mean changes in HbA1c from baseline to week 26 with liraglutide (n = 202) and placebo (n = 100) were - 0.98% and - 0.30%, respectively (estimated treatment difference [ETD]: -0.68% [95% CI: -0.89, -0.48]; P < 0.001). Mean body weight changes from baseline were - 2.81 versus -1.99 kg, respectively (ETD: -0.82 kg [95% CI: -1.73, 0.09]; P = 0.077); 51.8% of liraglutide-treated patients achieved HbA1c < 7.0% versus 23.2% receiving placebo (odds ratio: 5.1 [95% CI: 2.67, 9.87]; P < 0.001). More patients treated with liraglutide reported ≥1 treatment-emergent adverse events (66.3%) versus placebo (47.0%).
Conclusions: Liraglutide significantly improved glycaemic control compared with placebo in patients with type 2 diabetes, insufficiently controlled with SGLT2is with/without metformin, with no unexpected safety findings.
Keywords: glucagon-like peptide-1; liraglutide; randomized trial; sodium-glucose co-transporter-2 inhibitor.
© 2020 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
La.B. declares research support from Janssen, Lexicon, Merck, Novo Nordisk and Sanofi; speaker honoraria from Janssen, Novo Nordisk and Sanofi; consultant honoraria from AstraZeneca, Gilead, Janssen, Merck, Novo Nordisk and Sanofi. P.A.G.‐H. declares research support and honoraria from Amgen, Eli Lilly and Novo Nordisk. S.M.J. declares research support, speaker honorarium and advisory board membership from TOTALL Diabetes Hormone Institute. O.M. declares speaker's bureau honoraria and advisory board membership from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk and Sanofi; speaker's bureau honorarium from Bristol Myers Squibb. R.R. declares speaker's bureau membership from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi and Takeda; research support from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi; consulting fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Sanofi. U.F., M.S.K. and M.S.P. are employees of Novo Nordisk. Li.B. and J.N. declare no conflicts of interest.
Figures
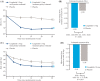
Figure 1
Change from baseline in HbA1c and body weight at week 26. (A) Mean HbA1c levels over 26 weeks, (B) HbA1c treatment effect at week 26 based on the in‐trial observation period, (C) mean body weight over 26 weeks, and (D) body weight treatment effect at week 26 based on the in‐trial observation period. Estimated treatment effect was calculated using treatment policy estimands with a pattern mixture model (PMM), which were based on the in‐trial observation period, including the effect of any rescue medication, regardless of whether patients prematurely discontinued trial product. Trial product estimands calculated using mixed model of repeated measurements (MMRMs) were based on the on‐treatment without rescue medication observation period. Error bars represent the standard error. ETD, estimated treatment difference
Figure 2
Proportion of patients reaching HbA1c targets and composite endpoints from baseline to 26 weeks (based on the in‐trial observation period)*. (A) Patients reaching an HbA1c target of <7.0%, (B) patients reaching an HbA1c target of ≤6.5%, (C) patients reaching an HbA1c target of <7.0% without severe or blood glucose (BG)‐confirmed symptomatic hypoglycaemia or body weight gain, and (D) HbA1c reduction of ≥1.0% with body weight loss ≥3%. *Treatment policy estimand using the in‐trial observation period including the effect of any rescue medication and regardless of whether subjects prematurely discontinued trial product. Hypoglycaemia classifications: severe or BG‐confirmed symptomatic: either severe according to the American Diabetes Association (ADA) definition (requiring assistance from another person) (33) or an episode accompanied by a plasma BG value of <3.1 mmol/L (56 mg/dL), with symptoms consistent with hypoglycaemia. n, number of patients experiencing at least one event; OR, odds ratio (with 95% confidence interval); %, percentage of patients experiencing at least one event
Similar articles
- Efficacy of liraglutide added to sodium-glucose cotransporter-2 inhibitors in type 2 diabetes, stratified by baseline characteristics: Post-hoc analysis of LIRA-ADD2SGLT2i.
Blonde L, Fainberg U, Kaltoft MS, Mosenzon O, Ramesh C, Rea R. Blonde L, et al. Diabetes Obes Metab. 2021 Oct;23(10):2234-2241. doi: 10.1111/dom.14464. Epub 2021 Jul 8. Diabetes Obes Metab. 2021. PMID: 34132018 Free PMC article. Clinical Trial. - Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial.
Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U, Jensen T, Jensen AK, Holst JJ, Tarnow L, Knop FK, Madsbad S, Andersen HU. Dejgaard TF, et al. Lancet Diabetes Endocrinol. 2016 Mar;4(3):221-232. doi: 10.1016/S2213-8587(15)00436-2. Epub 2015 Dec 3. Lancet Diabetes Endocrinol. 2016. PMID: 26656289 Clinical Trial. - Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial.
Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ; PIONEER 4 investigators. Pratley R, et al. Lancet. 2019 Jul 6;394(10192):39-50. doi: 10.1016/S0140-6736(19)31271-1. Epub 2019 Jun 8. Lancet. 2019. PMID: 31186120 Clinical Trial. - Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study.
Nauck M, Frid A, Hermansen K, Thomsen AB, During M, Shah N, Tankova T, Mitha I, Matthews DR. Nauck M, et al. Diabetes Obes Metab. 2013 Mar;15(3):204-12. doi: 10.1111/dom.12012. Epub 2012 Oct 11. Diabetes Obes Metab. 2013. PMID: 22985213 Review.
Cited by
- Switching to Versus Addition of Incretin-Based Drugs Among Patients With Type 2 Diabetes Taking Sodium-Glucose Cotransporter-2 Inhibitors.
Lau KTK, Wong CKH, Au ICH, Lau WCY, Man KKC, Chui CSL, Wong ICK. Lau KTK, et al. J Am Heart Assoc. 2022 Apr 5;11(7):e023489. doi: 10.1161/JAHA.121.023489. Epub 2022 Mar 24. J Am Heart Assoc. 2022. PMID: 35322676 Free PMC article. - The Efficacy and Safety of the Combination Therapy With GLP-1 Receptor Agonists and SGLT-2 Inhibitors in Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis.
Li C, Luo J, Jiang M, Wang K. Li C, et al. Front Pharmacol. 2022 Feb 4;13:838277. doi: 10.3389/fphar.2022.838277. eCollection 2022. Front Pharmacol. 2022. PMID: 35185588 Free PMC article. Review. - High-Dose Liraglutide and SGLT2 Inhibitor: A Promising Combination.
Chua MWJ. Chua MWJ. Clin Pract. 2021 Dec 21;12(1):1-7. doi: 10.3390/clinpract12010001. Clin Pract. 2021. PMID: 35076486 Free PMC article. - Combining GLP-1 Receptor Agonists and SGLT2 Inhibitors in Type 2 Diabetes Mellitus: A Scoping Review and Expert Insights for Clinical Practice Utilizing the Nominal Group Technique.
Yepes-Cortés CA, Cardenas-Moreno IC, Daza-Arnedo R, Feriz-Bonelo KM, Yama-Mosquera E, Ramirez-Rincón AH, Castillo-Barrios GA, Suarez-Rodriguez AF, Carreño-Jiménez J, Builes-Montaño CE. Yepes-Cortés CA, et al. Diabetes Ther. 2025 May;16(5):813-849. doi: 10.1007/s13300-025-01722-x. Epub 2025 Mar 24. Diabetes Ther. 2025. PMID: 40126829 Free PMC article. Review. - Safety and Efficacy of GLP-1 Receptor Agonists and SGLT2 Inhibitors Among Veterans With Type 2 Diabetes.
McCulley L, Hurren KM. McCulley L, et al. Fed Pract. 2022 Nov;39(Suppl 5):e0319. doi: 10.12788/fp.0319. Epub 2022 Nov 21. Fed Pract. 2022. PMID: 36923546 Free PMC article.
References
- American Diabetes Association . 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes‐2019. Diabetes Care. 2019;42:S90‐S102. - PubMed
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm ‐ 2019 Executive Summary. Endocr Pract. 2019;25:69‐100. - PubMed
- American Diabetes Association . 6. Glycemic Targets: Standards of Medical Care in Diabetes‐2019. Diabetes Care. 2019;42:S61‐S70. - PubMed
- Drucker DJ. The cardiovascular biology of glucagon‐like peptide‐1. Cell Metab. 2016;24:15‐30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical