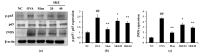Scrophularia koraiensis Nakai Attenuates Allergic Airway Inflammation via Suppression of NF-κB and Enhancement of Nrf2/HO-1 Signaling - PubMed (original) (raw)
Scrophularia koraiensis Nakai Attenuates Allergic Airway Inflammation via Suppression of NF-κB and Enhancement of Nrf2/HO-1 Signaling
Tae-Yang Jung et al. Antioxidants (Basel). 2020.
Abstract
Scrophularia koraiensis Nakai (Scrophulariaceae) is a medicinal herb that grows in Korea and which has been widely used to treat fever, edema, neuritis and laryngitis. Hence, we evaluated the anti-inflammatory and antioxidant effects of the ethanol extract (SKE) of S. koraiensis Nakai in an ovalbumin (OVA)-induced mouse model. We injected 20 μg of OVA with 2 mg of aluminum on day 0 and day 14 to induce allergic airway inflammation in six-week-old BALB/c mice, and mice were challenged with 1% OVA by nebulization for 1 h on days 21, 22, and 23. SKE was orally administered at 20 mg/kg and 40 mg/kg from day 18 to 23, and its effects were compared with those of montelukast treatment. SKE significantly reduced proinflammatory cytokines, inflammatory cell counts, immunoglobulin-E, and airway hyperresponsiveness during the OVA-induced allergic airway inflammation model; it also reduced airway inflammation and mucus production. In addition, SKE reduced the OVA-induced nuclear factor kappa B (NF-κB) phosphorylation in lung tissues while enhancing nuclear factor erythroid-derived 2-related factor (Nrf-2) and heme oxygenase-1 (HO-1) expression. In conclusion, SKE showed the protective effects on OVA-induced allergic airway inflammation via the suppression of NF-κB phosphorylation and the enhancement of the Nrf2/HO-1 signaling pathway. These results indicate that SKE is a potential therapeutic agent for allergic airway inflammation.
Keywords: Asthma; HO-1; NF-κB; Nrf2; Scrophularia koraiensis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
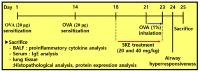
Figure 1
The experimental procedure.
Figure 2
Chromatogram of S. koraiensis at 200 nm (a) and 280 nm (b), UV spectra and MS spectra of peaks according to retention time (c), total ion chromatogram (TIC) and extracted ion chromatogram (XIC) (d).
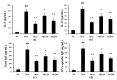
Figure 3
SKE (ethanol extract of S. koraiensis) reduced airway hyperresponsiveness (AHR) and inflammatory cell counts during ovalbumin (OVA)-induced allergic airway inflammation. The bronchoalveolar lavage fluid (BALF) was stained with a Diff-Quik agent for cell counting, and AHR was measured by using whole-body plethysmography. (a) AHR and (b) inflammatory cell counts in the BALF. NC (normal control), PBS (phosphate buffered saline) treatment and PBS sensitization/challenge; OVA, PBS treatment and OVA sensitization/challenge; Mon: montelukast treatment and OVA sensitization/challenge; SKE 20 and 40, SKE treatment (20 and 40 mg/kg, respectively) and OVA sensitization/challenge. Values are shown as the mean ± SD (n = 5). ## p < 0.01 versus NC; *,** p < 0.05 and 0.01 versus OVA.
Figure 4
SKE decreased proinflammatory cytokines and immunoglobulin E (IgE) during OVA-induced allergic airway inflammation. Interleukin (IL)-5, IL-13, total IgE, and OVA-specific IgE were determined with commercial ELISA kits. (a) IL-5, (b) IL-13, (c) total IgE, (d) OVA-specific IgE. NC, PBS treatment and PBS sensitization/challenge; OVA, PBS treatment and OVA sensitization/challenge; Mon: montelukast treatment and OVA sensitization/challenge; SKE 20 and 40, SKE treatment (20 and 40 mg/kg, respectively) and OVA sensitization/challenge. Values are shown as the mean ± SD (n = 5). ## p < 0.01 versus NC; *,** p < 0.05 and 0.01 versus OVA.
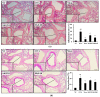
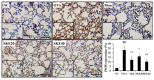
Figure 5
SKE attenuated airway inflammation and mucus production during OVA-induced allergic airway inflammation. Lung tissue samples were stained with hematoxylin and eosin (H and E) and Diff-Quik agents. (a) Airway inflammation and (b) mucus production. The quantitative analysis of airway inflammation and mucus production were measured by an image analyzer. NC, PBS treatment and PBS sensitization/challenge; OVA, PBS treatment and OVA sensitization/challenge; Mon: montelukast treatment and OVA sensitization/challenge; SKE 20 and 40, SKE treatment (20 and 40 mg/kg, respectively) and OVA sensitization/challenge. Scale bars indicate 50 μm. Values are shown as the mean ± SD (n = 5). ## p < 0.01 versus NC; *,** p < 0.05 and 0.01 versus OVA.
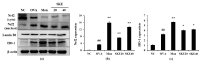
Figure 6
SKE decreased nuclear factor kappa B (NF-κB) phosphorylation and inducible nitric oxide synthase (iNOS) expression during OVA-induced allergic airway inflammation. NF-κB phosphorylation and iNOS expression were measured by western blotting. (a) Protein expression on gel, (b) relative p-p65/p65 expression value, and (c) relative iNOS expression value. NC, PBS treatment and PBS sensitization/challenge; OVA, PBS treatment and OVA sensitization/challenge; Mon: montelukast treatment and OVA sensitization/challenge; SKE 20 and 40, SKE treatment (20 and 40 mg/kg, respectively) and OVA sensitization/challenge. Values are shown as the mean ± SD (n = 5). ## p < 0.01 versus NC; *,** p < 0.05 and 0.01 versus OVA.
Figure 7
SKE reduced iNOS expression in the lungs during OVA-induced allergic airway inflammation. The expression of iNOS on lung tissue was determined by immunohistochemistry (IHC). NC, PBS treatment and PBS sensitization/challenge; OVA, PBS treatment and OVA sensitization/challenge; Mon: montelukast treatment and OVA sensitization/challenge; SKE 20 and 40, SKE treatment (20 and 40 mg/kg, respectively) and OVA sensitization/challenge. Scale bars indicate 50 μm. Values are shown as the mean ± SD (n = 5). ## p < 0.01 versus NC; *,** p < 0.05 and 0.01 versus OVA.
Figure 8
SKE enhanced Nrf2/HO-1 (nuclear factor erythroid-derived 2-related factor/heme oxygenase-1) signaling during the OVA-induced allergic airway inflammation model. The expression of Nrf2 and HO-1 were measured by western blotting. (a) Protein expression on gel, (b) relative nucleus/cytoplasm of Nrf2 expression value, (c) relative HO-1 expression value. NC, PBS treatment and PBS sensitization/challenge; OVA, PBS treatment and OVA sensitization/challenge; Mon: montelukast treatment and OVA sensitization/challenge; SKE 20 and 40, SKE treatment (20 and 40 mg/kg, respectively) and OVA sensitization/challenge. Values are shown as the mean ± SD (n = 5). ## p < 0.01 versus NC; *,** p < 0.05 and 0.01 versus OVA.
Figure 9
SKE reduced HO-1 expression in the lungs during OVA-induced allergic airway inflammation. The expression of HO-1 on lung tissue was determined by IHC. NC, PBS treatment and PBS sensitization/challenge; OVA, PBS treatment and OVA sensitization/challenge; Mon: montelukast treatment and OVA sensitization/challenge; SKE 20 and 40, SKE treatment (20 and 40 mg/kg, respectively) and OVA sensitization/challenge. Scale bars indicate 50 μm. Values are shown as the mean ± SD (n = 5). ## p < 0.01 versus NC; * p < 0.05 versus OVA.
Similar articles
- Cimicifugae Rhizoma Extract Attenuates Oxidative Stress and Airway Inflammation via the Upregulation of Nrf2/HO-1/NQO1 and Downregulation of NF-κB Phosphorylation in Ovalbumin-Induced Asthma.
Lim JO, Song KH, Lee IS, Lee SJ, Kim WI, Pak SW, Shin IS, Kim T. Lim JO, et al. Antioxidants (Basel). 2021 Oct 15;10(10):1626. doi: 10.3390/antiox10101626. Antioxidants (Basel). 2021. PMID: 34679759 Free PMC article. - Scrophularia buergeriana attenuates allergic inflammation by reducing NF-κB activation.
Shin NR, Lee AY, Song JH, Yang S, Park I, Lim JO, Jung TY, Ko JW, Kim JC, Lim KS, Lee MY, Shin IS, Kim JS. Shin NR, et al. Phytomedicine. 2020 Feb;67:153159. doi: 10.1016/j.phymed.2019.153159. Epub 2019 Dec 24. Phytomedicine. 2020. PMID: 31901567 - Protective effects of Angelica decursiva Franchet & Savatier on allergic responses through enhancement of Nrf2 and suppression of NF-kB/MMP-9 in ovalbumin-exposed mice.
Lee SJ, Lee AY, Pak SW, Kim WI, Yang YG, Lim JO, Chae SW, Cho YK, Kim JC, Moon BC, Seo YS, Shin IS. Lee SJ, et al. J Ethnopharmacol. 2024 Jan 10;318(Pt A):116863. doi: 10.1016/j.jep.2023.116863. Epub 2023 Jul 7. J Ethnopharmacol. 2024. PMID: 37423516 - Aloperine suppresses allergic airway inflammation through NF-κB, MAPK, and Nrf2/HO-1 signaling pathways in mice.
Wang C, Choi YH, Xian Z, Zheng M, Piao H, Yan G. Wang C, et al. Int Immunopharmacol. 2018 Dec;65:571-579. doi: 10.1016/j.intimp.2018.11.003. Epub 2018 Nov 8. Int Immunopharmacol. 2018. PMID: 30415164 - Immune Regulation of Heme Oxygenase-1 in Allergic Airway Inflammation.
Xia Z, Zhong W. Xia Z, et al. Antioxidants (Basel). 2022 Feb 26;11(3):465. doi: 10.3390/antiox11030465. Antioxidants (Basel). 2022. PMID: 35326116 Free PMC article. Review.
Cited by
- Interference with megalin expression/endocytic function by montelukast mitigates gentamicin nephrotoxicity: Downregulation of ClC-5 expression.
Azouz AA, Hanna DA, Abo-Saif AA, Anwar Shehata Messiha B. Azouz AA, et al. Saudi Pharm J. 2022 Feb;30(2):150-161. doi: 10.1016/j.jsps.2021.12.013. Epub 2022 Jan 4. Saudi Pharm J. 2022. PMID: 35528850 Free PMC article. - Cimicifugae Rhizoma Extract Attenuates Oxidative Stress and Airway Inflammation via the Upregulation of Nrf2/HO-1/NQO1 and Downregulation of NF-κB Phosphorylation in Ovalbumin-Induced Asthma.
Lim JO, Song KH, Lee IS, Lee SJ, Kim WI, Pak SW, Shin IS, Kim T. Lim JO, et al. Antioxidants (Basel). 2021 Oct 15;10(10):1626. doi: 10.3390/antiox10101626. Antioxidants (Basel). 2021. PMID: 34679759 Free PMC article. - An Integrative Study of Scrophularia takesimensis Nakai in an Ovalbumin-Induced Murine Model of Asthma: The Effect on T Helper 2 Cell Activation.
Seo YS, Song JH, Kim HS, Nam HH, Yang S, Choi G, Chae SW, Lee J, Jung B, Kim JS, Park I. Seo YS, et al. Pharmaceutics. 2024 Apr 12;16(4):529. doi: 10.3390/pharmaceutics16040529. Pharmaceutics. 2024. PMID: 38675190 Free PMC article. - Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials.
Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Nur H, Ismail AF, Sharif S, RamaKrishna S, Berto F. Parham S, et al. Antioxidants (Basel). 2020 Dec 21;9(12):1309. doi: 10.3390/antiox9121309. Antioxidants (Basel). 2020. PMID: 33371338 Free PMC article. Review. - Loranthus tanakae Franch. and Sav. Attenuates Respiratory Inflammation Caused by Asian Sand Dust.
Lee SJ, Pak SW, Lee AY, Kim WI, Chae SW, Cho YK, Ko JW, Kim TW, Kim JC, Moon BC, Seo YS, Shin IS. Lee SJ, et al. Antioxidants (Basel). 2024 Mar 29;13(4):419. doi: 10.3390/antiox13040419. Antioxidants (Basel). 2024. PMID: 38671867 Free PMC article.
References
LinkOut - more resources
Full Text Sources