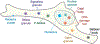Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions - PubMed (original) (raw)
Review
Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions
Jeong-Mo Choi et al. Annu Rev Biophys. 2020.
Abstract
Many biomolecular condensates appear to form via spontaneous or driven processes that have the hallmarks of intracellular phase transitions. This suggests that a common underlying physical framework might govern the formation of functionally and compositionally unrelated biomolecular condensates. In this review, we summarize recent work that leverages a stickers-and-spacers framework adapted from the field of associative polymers for understanding how multivalent protein and RNA molecules drive phase transitions that give rise to biomolecular condensates. We discuss how the valence of stickers impacts the driving forces for condensate formation and elaborate on how stickers can be distinguished from spacers in different contexts. We touch on the impact of sticker- and spacer-mediated interactions on the rheological properties of condensates and show how the model can be mapped to known drivers of different types of biomolecular condensates.
Keywords: biomolecular condensates; phase separation; phase transition; stickers and spacers.
Figures
Figure 1:. Overview of cellular bodies that are well-described as condensates.
These bodies include large well-studied structures such as the nucleolus, nuclear speckles, and P-bodies, but also smaller assemblies including those such as signaling granules, receptor clusters and DNA damage foci. The size of assemblies in this schematic are not to scale.
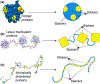
Figure 2:. Schematic of different types of stickers and spacers for different systems.
(a) For folded proteins we can map stickers and spacers to the patchy colloid formalism. (b) For linear multivalent proteins we can broadly map stickers as folded binding domains while spacers are flexible linkers that connected domains (c) For intrinsically disordered proteins stickers may be single residues, short linear motifs, or some combination of both.
Figure 3:. Schematic of sticker patterning and effective solvation volume.
(a) Three distinct sequences with identical numbers of sticker residues distributed in different arrangements. As sticker residues are clustered together, the effective sticker identity may change, such that as the number of stickers decrease the strength of each individual sticker increases. There are likely complex non-linearities in this behavior, such that the schematic here should be taken only as a qualitative description of this phenomenon. B Physical manifestation of the effective solvation volume for linkers. A positive effective solvation volume is associated with expanded and highly expanded linkers while a negative effective solvation volume leads to a collapsed and self-interacting linker. An effective solvation volume of zero implies ideal chain behavior.
Figure 4:. Four examples of multiphase assemblies formed from different components.
(a) Three-phase nucleoli assembly is readily reproduced using a simple stickers-and-spacers model, as shown by Feric et al. (b) Various distinct types of nuclear speckle architecture can also be recapitulated in a similar manner, as shown by Fei et al. (c) MEG-3 and PGL-3 form distinct phases in P-granules and in vitro, as demonstrated by Putnam et al. (d) A simple four component system (solvent, proline-arginine dipeptides, polyadenosine, polycytosine) forms two distinct dense phases due to distinct sticker-sticker strengths, as described by Boeynaems et al.
Figure 5:
(a) General model for emergent stickers formed through oligomerization. Monomeric species might lack the requisite valence to drive condensates, but oligomerization through a defined interface gives rise to multivalence of emergent stickers that drive condensate formation. (b) A schematized version of condensate regulation in the context of ARF19. The PB1 oligomerization domain drives assembly via an electrostatically mediated binding surface. Neutralization of a lysine residues abrogates oligomerization and consequently prevents condensate formation. (c) For a system that gives rise to emergent stickers, condensate formation can be regulated at two levels. An effectively binary regulation that dictates whether oligomerization occurs (modulation of valence), and a second level in which the strength of emergent stickers can be altered. Note that temporally the order in which these levels of modulation occur is irrelevant. As a tangible example, in a scenario in which IDRs phosphorylation weakens the strength of emergent stickers, the act of phosphorylation could happen before or after oligomerization. The binding of ligands, which could include other proteins, nucleic acids, small molecules, may either lead to a conformational transition that allows homotypic oligomerization or itself could drive heterotypic assembly. In principle, multiple nested layers of assembly via orthogonal interaction modes provide the foundations for arbitrarily complex regulation.
Similar articles
- Ligand effects on phase separation of multivalent macromolecules.
Ruff KM, Dar F, Pappu RV. Ruff KM, et al. Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2017184118. doi: 10.1073/pnas.2017184118. Proc Natl Acad Sci U S A. 2021. PMID: 33653957 Free PMC article. - LASSI: A lattice model for simulating phase transitions of multivalent proteins.
Choi JM, Dar F, Pappu RV. Choi JM, et al. PLoS Comput Biol. 2019 Oct 21;15(10):e1007028. doi: 10.1371/journal.pcbi.1007028. eCollection 2019 Oct. PLoS Comput Biol. 2019. PMID: 31634364 Free PMC article. - An Introduction to the Stickers-and-Spacers Framework as Applied to Biomolecular Condensates.
Ginell GM, Holehouse AS. Ginell GM, et al. Methods Mol Biol. 2023;2563:95-116. doi: 10.1007/978-1-0716-2663-4_4. Methods Mol Biol. 2023. PMID: 36227469 - Molecular and environmental determinants of biomolecular condensate formation.
Villegas JA, Heidenreich M, Levy ED. Villegas JA, et al. Nat Chem Biol. 2022 Dec;18(12):1319-1329. doi: 10.1038/s41589-022-01175-4. Epub 2022 Nov 18. Nat Chem Biol. 2022. PMID: 36400992 Review. - Making the Case for Disordered Proteins and Biomolecular Condensates in Bacteria.
Cohan MC, Pappu RV. Cohan MC, et al. Trends Biochem Sci. 2020 Aug;45(8):668-680. doi: 10.1016/j.tibs.2020.04.011. Epub 2020 May 23. Trends Biochem Sci. 2020. PMID: 32456986 Review.
Cited by
- Sequence determinants of in cell condensate morphology, dynamics, and oligomerization as measured by number and brightness analysis.
Emenecker RJ, Holehouse AS, Strader LC. Emenecker RJ, et al. Cell Commun Signal. 2021 Jun 5;19(1):65. doi: 10.1186/s12964-021-00744-9. Cell Commun Signal. 2021. PMID: 34090478 Free PMC article. - Wnt target gene activation requires β-catenin separation into biomolecular condensates.
Stewart RA, Ding Z, Jeon US, Goodman LB, Tran JJ, Zientko JP, Sabu M, Cadigan KM. Stewart RA, et al. PLoS Biol. 2024 Sep 24;22(9):e3002368. doi: 10.1371/journal.pbio.3002368. eCollection 2024 Sep. PLoS Biol. 2024. PMID: 39316611 Free PMC article. - Direct Observation of "Elongated" Conformational States in α-Synuclein upon Liquid-Liquid Phase Separation.
Ubbiali D, Fratini M, Piersimoni L, Ihling CH, Kipping M, Heilmann I, Iacobucci C, Sinz A. Ubbiali D, et al. Angew Chem Int Ed Engl. 2022 Nov 14;61(46):e202205726. doi: 10.1002/anie.202205726. Epub 2022 Oct 17. Angew Chem Int Ed Engl. 2022. PMID: 36115020 Free PMC article. - Higher-order organization of biomolecular condensates.
Fare CM, Villani A, Drake LE, Shorter J. Fare CM, et al. Open Biol. 2021 Jun;11(6):210137. doi: 10.1098/rsob.210137. Epub 2021 Jun 16. Open Biol. 2021. PMID: 34129784 Free PMC article. Review. - Direct prediction of intrinsically disordered protein conformational properties from sequence.
Lotthammer JM, Ginell GM, Griffith D, Emenecker RJ, Holehouse AS. Lotthammer JM, et al. Nat Methods. 2024 Mar;21(3):465-476. doi: 10.1038/s41592-023-02159-5. Epub 2024 Jan 31. Nat Methods. 2024. PMID: 38297184 Free PMC article.
References
- Bickmore WA. 2013. Annual Reviews in Genomics Human Genetics 14:67–84 - PubMed
- Lee H, DeLoache WC, Dueber JE. 2012. Metabolic engineering 14:242–51 - PubMed
- Razin SV, Gavrilov AA, Yarovaya OV. 2010. Biochemistry. Biokhimiia 75:1307–15 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources