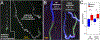Antagonistic Inhibitory Circuits Integrate Visual and Gravitactic Behaviors - PubMed (original) (raw)
Antagonistic Inhibitory Circuits Integrate Visual and Gravitactic Behaviors
Michaela Bostwick et al. Curr Biol. 2020.
Abstract
Larvae of the tunicate Ciona intestinalis possess a central nervous system of 177 neurons. This simplicity has facilitated the generation of a complete synaptic connectome. As chordates and the closest relatives of vertebrates, tunicates promise insight into the organization and evolution of vertebrate nervous systems. Ciona larvae have several sensory systems, including the ocellus and otolith, which are sensitive to light and gravity, respectively. Here, we describe circuitry by which these two are integrated into a complex behavior: the rapid reorientation of the body followed by upward swimming in response to dimming. Significantly, the gravity response causes an orienting behavior consisting of curved swims in downward-facing larvae but only when triggered by dimming. In contrast, the majority of larvae facing upward do not respond to dimming with orientation swims-but instead swim directly upward. Under constant light conditions, the gravity circuit appears to be inoperable, and both upward and downward swims were observed. Using connectomic and neurotransmitter data, we propose a circuit model that can account for these behaviors. The otolith consists of a statocyst cell and projecting excitatory sensory neurons (antenna cells). Postsynaptic to the antenna cells are a group of inhibitory primary interneurons, the antenna relay neurons (antRNs), which then project asymmetrically to the right and left motor units, thereby mediating curved orientation swims. Also projecting to the antRNs are inhibitory photoreceptor relay interneurons. These interneurons appear to antagonize the otolith circuit until they themselves are inhibited by photoreceptors in response to dimming, thus providing a triggering circuit.
Keywords: Ciona; connectome; gravitaxis; multisensory integration; neural circuit.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
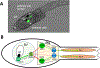
Figure 1.. Ciona gravitaxis circuit.
A. Twenty-five hours post-fertilization (hpf) larva expressing Kaede fluorescent protein from an injected plasmid containing the vesicular glutamate transporter (VGLUT) cis-regulatory element. The antenna cells are green. B. Simplified minimal gravitaxis circuit. Cells of similar type and connectivity (circles) and their synaptic connections (lines) were combined based on connectome data from a 21-hpf larva. Cell types includes the two antenna cells (Ant), the eleven antenna relay neurons (Ant RN), the three left and right motoganglion interneurons (MGIN L and MGIN R, respectively), the five left and right motor neurons (MN L and MN R, respectively), and the 18 left and right muscles (Mu L and Mu R, respectively). Likely neurotransmitter types derived from in situ hybridization studies are indicated (Glut: glutamate; GABA: gamma-aminobutyric acid; ACh: acetylcholine), as well as putative excitatory (green), inhibitory (red) and electrical (gap junction) synapses (black). Other abbreviations: OT: otolith cell, BV: brain vesicle; MG: motor ganglion.
Figure 2. Ciona gravitaxis behavior.
A. Imaging set-up for recording gravitaxis behavior. B. Five-second projection images of 21- and 25-hour post fertilization (hpf) larvae swimming in response to the turning off of visible light (lights-off condition; swim traces in white on dark background). The beginning position of larvae is indicated in green and their position after 5 seconds is in red. Up is towards the top of the images. The orange arrow indicates a larva swimming downward and the blue arrow indicates a larva swimming sideways. C. Quantified swim trajectory data for swims under the indicated conditions and developmental ages. Percentages of larvae within the categorized trajectories [(UP, DOWN and SIDE (sideways)] are shown. Values are from the averages of nine videos (± standard deviation), with 20 larvae assessed per video. D. Matrix comparing the compiled swim data from different conditions (numbered as in C) for significant differences. (*: p < 0.05; ***: p<0.005; Wilcoxon Signed Rank Test). E. Quantified swim trajectory data for 25-hpf larvae in response to 6- and 10-fold dimming of visible light. F. lights-off induced swims are more tortuous at 21-hpf than at 25-hpf (***: p <0.005; Wilcoxon Ranksum Test).
Figure 3.. Reorientation Behavior.
A. Response of stationary up-facing and down-facing larvae to lights-off. The initial position of larvae are shown in green, and swims are projected over time in white. B. Response of swimming larvae to lights-off. Projection images of larvae show the trajectory before lights-off (green) and after (blue). Magenta marks the first frame of lights-off. C. Both upward and downward swimmers accelerate at lights-off. Swim speeds before and after lights-off are indicated (*: p<0.05; ***: p<0.005; T-test).
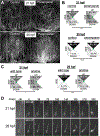
Figure 4.. Gravitaxis response is inhibitory.
A. Imaging set-up used to record behavioral response of Ciona larvae to being rotated with respect to gravity. B. Results of rotation experiments. The top row shows up-facing larvae immediately before rotation, immediately after rotation, or after visible light is turned off. The bottom row shows the same conditions, but with down-facing larvae. The results show the percentage of larvae in 10-second windows displaying short, long, or no swims. For comparisons between groups, swim categories were assigned values and assessed with the Wilcoxon Signed Rank Test (*: p<0.05; *** p<0.005). C. Five-second projection images showing swims in control and picrotoxin-treated larvae. Swims appear as white lines. Up is toward the top of the image. D. Quantification of swim trajectories in control and picrotoxin-treated larvae, analyzed as in Figure 2C.
Figure 5.. Gravitaxis comparisons in 21- and 25-hours post fertilization larvae.
A. Control (DMSO vehicle) and perampanel-treated larvae. Shown are 10-second projections of swims following lights-off. Larvae were assessed at 21- and 25 hours post fertilization (hpf). B. Quantification of swim trajectory in control and perampanel-treated larvae. Shown are the swim trajectories [UP, DOWN or SIDE (sideways)] in five second windows following lights-off. The values are averages from 10 time-lapse videos (20 larvae analyzed per video) plus or minus standard deviations. For comparisons between groups, swim directions were assigned values and assessed with the Wilcoxon Signed Rank Test. C. Swim directions following lights-off in wild-type and homozygous pristine mutants. The analysis was performed as in B (*: p<0.05; *** p<0.005). D. Frames from time-lapse recordings showing representative reaction times to lights-off at 21- and 25-hpf. Times in milliseconds (msec) are indicated.
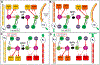
Figure 6.. Model of Gravitaxis Circuitry.
Each panel shows both a cartoon larva with the relative activation of the muscle cells shown from low (yellow), to intermediate (orange) and high (red), and a diagram of the gravitaxis and photoreceptor circuits. In the circuit diagrams, proposed inhibitory synaptic projections are in red, excitatory are in green, and electrical in black. A. Behavior and circuitry of a down-facing larva before (left), and after (right) visible light being turned off (lights-off). Asymmetric inhibition from the antRNs leads to asymmetric L/R muscle activation and turning (green arrow). B. Behavior and circuitry of downward swimming larva. Note that inhibition of the antRNs by the pr-AMG RNs allows for symmetrical activation of muscles (orange) before lights-off. C. Behavior and circuitry of up-facing larva. Note that the antenna cells are inactive when the larva is in this position. At lights-off the larvae can go directly to symmetric swim. D. Behavior and circuitry of upward swimming larva. In the model release of inhibition by the pr-AMG RNs on the MGINs at lights-out leads to acceleration. Abbreviations: PR-II: photoreceptor group II; pr-AMG RN: photoreceptor-ascending motor ganglion relay neurons; Ant: antenna cells; antRN: antenna relay neurons; MGIN: motor ganglion interneurons; R: right: L: left.
Comment in
- Neurobiology: Swimming at the Intersection of Light and Gravity.
Popsuj S, Stolfi A. Popsuj S, et al. Curr Biol. 2020 Feb 24;30(4):R171-R174. doi: 10.1016/j.cub.2019.12.034. Curr Biol. 2020. PMID: 32097645
Similar articles
- A single oscillating proto-hypothalamic neuron gates taxis behavior in the primitive chordate Ciona.
Chung J, Newman-Smith E, Kourakis MJ, Miao Y, Borba C, Medina J, Laurent T, Gallean B, Faure E, Smith WC. Chung J, et al. Curr Biol. 2023 Aug 21;33(16):3360-3370.e4. doi: 10.1016/j.cub.2023.06.080. Epub 2023 Jul 24. Curr Biol. 2023. PMID: 37490920 Free PMC article. - Circuit Homology between Decussating Pathways in the Ciona Larval CNS and the Vertebrate Startle-Response Pathway.
Ryan K, Lu Z, Meinertzhagen IA. Ryan K, et al. Curr Biol. 2017 Mar 6;27(5):721-728. doi: 10.1016/j.cub.2017.01.026. Epub 2017 Feb 16. Curr Biol. 2017. PMID: 28216318 - Disruption of left-right axis specification in Ciona induces molecular, cellular, and functional defects in asymmetric brain structures.
Kourakis MJ, Bostwick M, Zabriskie A, Smith WC. Kourakis MJ, et al. BMC Biol. 2021 Jul 13;19(1):141. doi: 10.1186/s12915-021-01075-4. BMC Biol. 2021. PMID: 34256748 Free PMC article. - [Does Paramecium sense gravity?].
Mogami Y, Ishii J, Baba SA. Mogami Y, et al. Biol Sci Space. 1995 Mar;9(1):17-35. Biol Sci Space. 1995. PMID: 11541872 Review. Japanese. - Central circuits controlling locomotion in young frog tadpoles.
Roberts A, Soffe SR, Wolf ES, Yoshida M, Zhao FY. Roberts A, et al. Ann N Y Acad Sci. 1998 Nov 16;860:19-34. doi: 10.1111/j.1749-6632.1998.tb09036.x. Ann N Y Acad Sci. 1998. PMID: 9928299 Review.
Cited by
- Developmental Table and Three-Dimensional Embryological Image Resource of the Ascidian Ascidiella aspersa.
Funakoshi HM, Shito TT, Oka K, Hotta K. Funakoshi HM, et al. Front Cell Dev Biol. 2021 Dec 17;9:789046. doi: 10.3389/fcell.2021.789046. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34977032 Free PMC article. - Evolution and development of complex eyes: a celebration of diversity.
Koenig KM, Gross JM. Koenig KM, et al. Development. 2020 Oct 13;147(19):dev182923. doi: 10.1242/dev.182923. Development. 2020. PMID: 33051250 Free PMC article. Review. - The Degenerate Tale of Ascidian Tails.
Fodor ACA, Powers MM, Andrykovich K, Liu J, Lowe EK, Brown CT, Di Gregorio A, Stolfi A, Swalla BJ. Fodor ACA, et al. Integr Comp Biol. 2021 Sep 8;61(2):358-369. doi: 10.1093/icb/icab022. Integr Comp Biol. 2021. PMID: 33881514 Free PMC article. Review. - Deep homologies in chordate caudal central nervous systems.
Kourakis MJ, Ryan K, Newman-Smith ED, Meinertzhagen IA, Smith WC. Kourakis MJ, et al. bioRxiv [Preprint]. 2024 Jun 4:2024.06.03.597227. doi: 10.1101/2024.06.03.597227. bioRxiv. 2024. PMID: 38895365 Free PMC article. Preprint. - Development and circuitry of the tunicate larval Motor Ganglion, a putative hindbrain/spinal cord homolog.
Piekarz KM, Stolfi A. Piekarz KM, et al. J Exp Zool B Mol Dev Evol. 2024 May;342(3):200-211. doi: 10.1002/jez.b.23221. Epub 2023 Sep 7. J Exp Zool B Mol Dev Evol. 2024. PMID: 37675754 Free PMC article. Review.
References
- Häder D-P, and Hemmersbach R. (2017). Gravitaxis in Euglena In Euglena:Biochemistry, Cell and Molecular Biology Advances in Experimental Medicine and Biology, Schwartzbach SD and Shigeoka S, eds. (Cham: Springer International Publishing; ), pp. 237–266. Available at: 10.1007/978-3-319-54910-1_12 [Accessed September 27, 2019]. - DOI - PubMed
- Nakamura M, Nishimura T, and Morita MT (2019). Gravity sensing and signal conversion in plant gravitropism. J. Exp. Bot 70, 3495–3506. - PubMed
- Beraneck M, Lambert FM, and Sadeghi SG (2014). Chapter 15 - Functional Development of the Vestibular System: Sensorimotor Pathways for Stabilization of Gaze and Posture In Development of Auditory and Vestibular Systems, Romand R. and Varela-Nieto I, eds. (San Diego: Academic Press; ), pp. 449–487. Available at: http://www.sciencedirect.com/science/article/pii/B9780124080881000154 [Accessed September 27, 2019].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials