Xylooligosaccharide Modulates Gut Microbiota and Alleviates Colonic Inflammation Caused by High Fat Diet Induced Obesity - PubMed (original) (raw)
Xylooligosaccharide Modulates Gut Microbiota and Alleviates Colonic Inflammation Caused by High Fat Diet Induced Obesity
Yanquan Fei et al. Front Physiol. 2020.
Abstract
Obesity leads to colonic inflammation and may increase the risk of colorectal cancer. Xylooligosaccharide (XOS) exhibits strong antioxidant and excellent antibacterial properties, and can be utilized by gut microbes to maintain the ecological balance of the intestinal tract. In this study, we explored how XOS modulates the microbiota and regulates high fat diet (HFD) induced inflammation. We measured the changes in body weight and visceral coefficients in rats fed a high-fat diet. We also measured the expression levels of inflammatory factors in the plasma and colonic tissues of the rats using the enzyme-linked immunosorbent assay and real-time quantitative polymerase chain reaction. We analyzed the composition of fecal microorganisms and short chain fatty acid (SCFA) content using 16S rDNA and GC-MS. We found that XOS significantly counteracted HFD induced weight gain. Moreover, the plasma levels of monocyte chemoattractant protein-1, tumor necrosis factor (TNF-α) and lipopolysaccharide decreased in the XOS-treated rats. XOS treatment decreased TNF-α mRNA expression and increased occludin mRNA expression in the rat colon. We observed a reduction in the overall microbial abundance in the feces of the XOS-treated rats, although the proportion of Bacteroidetes/Firmicutes increased significantly and the number of beneficial bacteria increased in the form of dominant microbes. We found that both SCFA-producing bacteria and SCFA content increased in the gut of the XOS-treated rats. We identified a correlation between the abundance of Prevotella and Paraprevotella and SCFA content. Taken together, we propose that XOS can alleviate colonic inflammation by regulating gut microbial composition and enhancing SCFA content in the gut.
Keywords: SCFA; colon inflammation; gut microbiota; high-fat diet; obesity.
Copyright © 2020 Fei, Wang, Pang, Wang, Zhu, Xie, Lan and Wang.
Figures
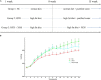
FIGURE 1
Effects of HFD and XOS on body weight of rats. (A) The process of treatment, (B) body weight changes. Data are given as mean ± SD (n = 10), a, b, cmean values with different letters are significantly different from each other (P < 0.05).
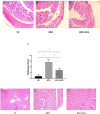
FIGURE 2
Representative colon HE staining results. The colonic tissues of NC rats (A), HFD rats (B), (a: crypt injury, b: intestinal edema of the colonic mucosa, and c: intestinal edema of the colonic mucosa), and HFD + XOS rats (C) were imaged at 20× magnification. (D) Histological score. The liver tissues of NC rats (E), HFD rats (F), and HFD + XOS rats (G) were imaged at 20× magnification. Data are given as mean ± SD (n = 10), *P < 0.05.
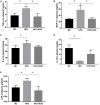
FIGURE 3
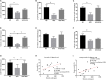
Impact of XOS treatment on the expression of plasma inflammatory cytokines. The levels of TNF-α (A), MCP-1 (B), IL-6 (C), IL-10 (D), and LPS (E) were determined. Data are given as mean ± SD (n = 10), *P < 0.05.
FIGURE 4
Impact of XOS treatment on the expression of colonic inflammatory cytokines. The relative gene expression levels for TNF-α (A), MCP-1 (B), OCLN (C), IL-10 (D), and IL-6 (E) were determined using RT-PCR. Data are given as mean ± SD (n = 10), *P < 0.05.
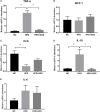
FIGURE 5
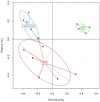
Principal component analysis (PCA) score plot based on the OTU abundance. NC, normal control group; HFD, high fat diet group; HFD + XOS, XOS plus high fat diet (n = 5).
FIGURE 6
Microbial composition analysis. Taxonomic composition of the fecal bacterial communities at the phylum level (A). Taxonomic composition of the fecal bacterial communities at the genus level (B). Data are given as mean ± SD (n = 5), *P < 0.05.
FIGURE 7
Determination of fecal SCFA content and its correlation with abundance of microorganisms. The levels of acetic acid (A), propionic acid (B), isobutyric acid (C), butyric acid (D), isovaleric acid (E), and valeric acid (F) were determined. The correlation between Prevotella and acetic acid (G), the correlation between Bacteroidetes and acetic acid (H) and the correlation between Lactobacillus and acetic acid (I) were analyzed. Data are given as mean ± SD (n = 5), *P < 0.05.
Similar articles
- Xylooligosaccharide attenuates lipopolysaccharide-induced intestinal injury in piglets via suppressing inflammation and modulating cecal microbial communities.
Wang X, Xiao K, Yu C, Wang L, Liang T, Zhu H, Xu X, Liu Y. Wang X, et al. Anim Nutr. 2021 Sep;7(3):609-620. doi: 10.1016/j.aninu.2020.11.008. Epub 2021 Mar 22. Anim Nutr. 2021. PMID: 34377847 Free PMC article. - Colonic aberrant crypt formation accompanies an increase of opportunistic pathogenic bacteria in C57BL/6 mice fed a high-fat diet.
Zeng H, Ishaq SL, Liu Z, Bukowski MR. Zeng H, et al. J Nutr Biochem. 2018 Apr;54:18-27. doi: 10.1016/j.jnutbio.2017.11.001. Epub 2017 Nov 10. J Nutr Biochem. 2018. PMID: 29223827 - Effects of shenling baizhu powder herbal formula on intestinal microbiota in high-fat diet-induced NAFLD rats.
Zhang Y, Tang K, Deng Y, Chen R, Liang S, Xie H, He Y, Chen Y, Yang Q. Zhang Y, et al. Biomed Pharmacother. 2018 Jun;102:1025-1036. doi: 10.1016/j.biopha.2018.03.158. Epub 2018 Apr 5. Biomed Pharmacother. 2018. PMID: 29710519 - Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation.
Akhtar M, Chen Y, Ma Z, Zhang X, Shi D, Khan JA, Liu H. Akhtar M, et al. Anim Nutr. 2021 Dec 29;8:350-360. doi: 10.1016/j.aninu.2021.11.005. eCollection 2022 Mar. Anim Nutr. 2021. PMID: 35510031 Free PMC article. Review. - The Therapeutic Effect of SCFA-Mediated Regulation of the Intestinal Environment on Obesity.
You H, Tan Y, Yu D, Qiu S, Bai Y, He J, Cao H, Che Q, Guo J, Su Z. You H, et al. Front Nutr. 2022 May 17;9:886902. doi: 10.3389/fnut.2022.886902. eCollection 2022. Front Nutr. 2022. PMID: 35662937 Free PMC article. Review.
Cited by
- Ling-Gui-Zhu-Gan decoction ameliorates nonalcoholic fatty liver disease via modulating the gut microbiota.
Chen L-p, Zhang L-f, Liu S, Hua H, Zhang L, Liu B-c, Wang R-r. Chen L-p, et al. Microbiol Spectr. 2024 Jun 4;12(6):e0197923. doi: 10.1128/spectrum.01979-23. Epub 2024 Apr 22. Microbiol Spectr. 2024. PMID: 38647315 Free PMC article. - Probiotics, prebiotics, and postbiotics in health and disease.
Ji J, Jin W, Liu SJ, Jiao Z, Li X. Ji J, et al. MedComm (2020). 2023 Nov 4;4(6):e420. doi: 10.1002/mco2.420. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 37929014 Free PMC article. Review. - Dynamic changes of fecal microbiota in a weight-change model of Bama minipigs.
Zeng B, Chen L, Kong F, Zhang C, Chen L, Qi X, Chai J, Jin L, Li M. Zeng B, et al. Front Microbiol. 2023 Oct 20;14:1239847. doi: 10.3389/fmicb.2023.1239847. eCollection 2023. Front Microbiol. 2023. PMID: 37928663 Free PMC article. - Non-digestible oligosaccharides-based prebiotics to ameliorate obesity: Overview of experimental evidence and future perspectives.
Divyashri G, Karthik P, Murthy TPK, Priyadarshini D, Reddy KR, Raghu AV, Vaidyanathan VK. Divyashri G, et al. Food Sci Biotechnol. 2023 Aug 9;32(14):1993-2011. doi: 10.1007/s10068-023-01381-3. eCollection 2023 Dec. Food Sci Biotechnol. 2023. PMID: 37860742 Free PMC article. Review. - Gut microbiota changes in horses with Chlamydia.
Jin Y, Li W, Ba X, Li Y, Wang Y, Zhang H, Li Z, Zhou J. Jin Y, et al. BMC Microbiol. 2023 Sep 2;23(1):246. doi: 10.1186/s12866-023-02986-8. BMC Microbiol. 2023. PMID: 37660043 Free PMC article.
References
- Alex S., Lange K., Amolo T., Grinstead J. S., Haakonsson A. K., Szalowska E., et al. (2013). Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor gamma. Mol. Cell Biol. 33 1303–1316. 10.1128/mcb.00858-812 - DOI - PMC - PubMed
- Childs C. E., Roytio H., Alhoniemi E., Fekete A. A., Forssten S. D., Hudjec N., et al. (2014). Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Br. J. Nutr. 111 1945–1956. 10.1017/s0007114513004261 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous