Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment - PubMed (original) (raw)
Review
Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment
Elizabeth Katherine Batchelor et al. J Am Soc Nephrol. 2020 Mar.
Abstract
Anemia is a complication that affects a majority of individuals with advanced CKD. Although relative deficiency of erythropoietin production is the major driver of anemia in CKD, iron deficiency stands out among the mechanisms contributing to the impaired erythropoiesis in the setting of reduced kidney function. Iron deficiency plays a significant role in anemia in CKD. This may be due to a true paucity of iron stores (absolute iron deficiency) or a relative (functional) deficiency which prevents the use of available iron stores. Several risk factors contribute to absolute and functional iron deficiency in CKD, including blood losses, impaired iron absorption, and chronic inflammation. The traditional biomarkers used for the diagnosis of iron-deficiency anemia (IDA) in patients with CKD have limitations, leading to persistent challenges in the detection and monitoring of IDA in these patients. Here, we review the pathophysiology and available diagnostic tests for IDA in CKD, we discuss the literature that has informed the current practice guidelines for the treatment of IDA in CKD, and we summarize the available oral and intravenous (IV) iron formulations for the treatment of IDA in CKD. Two important issues are addressed, including the potential risks of a more liberal approach to iron supplementation as well as the potential risks and benefits of IV versus oral iron supplementation in patients with CKD.
Keywords: anemia; chronic kidney disease; iron.
Copyright © 2020 by the American Society of Nephrology.
Figures
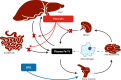
Figure 1.
Mechanisms of Anemia in CKD. HIF-PHD, HIF prolyl-hydroxylase domain–containing proteins.
Figure 2.
Iron metabolism is a tightly regulated process. Iron is absorbed in the gut and bound to soluble transferrin. Iron is then moved to storage in the bone marrow and used for erythropoiesis. Additional stores are repleted by macrophage uptake of iron from RBC destruction. EPO induces RBC production, leading to the mobilization of iron stores from the bone marrow. Hepcidin, which is produced by the liver and often stimulated by inflammation, leads to decreased iron uptake from the gut and decreased mobilization of iron stores. Fe-Tf, iron-bound transferrin.
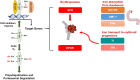
Figure 3.
Hypoxia signaling controls erythropoiesis by coordinating EPO synthesis with the expression of genes involved in iron metabolism. Under well oxygenated conditions, the three oxygen-labile HIF-α subunits (HIF-1_α_, HIF-2_α_, and HIF-3_α_) are hydroxylated at specific proline (Pro) residues by PHD enzymes. Prolyl hydroxylation targets HIF-α proteins for ubiquitination by the von Hippel-Lindau (pVHL)-E3-ubiquitin ligase complex with subsequent proteasomal degradation. Under conditions of reduced PHD activity (for example hypoxia or pharmacologic inhibition), HIF-α escapes hydroxylation and translocates to the nucleus, where it forms a heterodimer with the constitutively expressed HIF-β subunit. The effective HIF-α/HIF-β complex activates the transcription genes whose promoters contain hypoxia response elements. The HIF-mediated activation of transcription requires the recruitment of coactivators such as p300/CREB-binding protein. An additional layer of regulation is due to factor-inhibiting HIF (FIH), which hydroxylates a specific asparagine (Asn) residue abrogating transcriptional cofactor recruitment. HIF-α stabilization results in the activation of genes in diverse biologic processes. For instance, HIF-2_α_ induces EPO production from renal peritubular fibroblasts and hepatocytes, promoting erythroid progenitors’ cell viability, proliferation, and differentiation through EPO receptor (EPOR) signaling. Genes expressed in duodenum, duodenal cytochrome b reductase (DCYTB) and divalent metal transporter-1 (DMT1) along with ferroportin (FPN) are activated by HIF-2, increasing iron uptake while HIF signaling also controls the expression of iron transport genes transferrin (Tf) and transferrin receptor (TfR). O2, oxygen; OH, hydroxide.
Similar articles
- Impact of Inflammation on Ferritin, Hepcidin and the Management of Iron Deficiency Anemia in Chronic Kidney Disease.
Ueda N, Takasawa K. Ueda N, et al. Nutrients. 2018 Aug 27;10(9):1173. doi: 10.3390/nu10091173. Nutrients. 2018. PMID: 30150549 Free PMC article. Review. - Iron Deficiency Anemia in Chronic Kidney Disease.
Gafter-Gvili A, Schechter A, Rozen-Zvi B. Gafter-Gvili A, et al. Acta Haematol. 2019;142(1):44-50. doi: 10.1159/000496492. Epub 2019 Apr 10. Acta Haematol. 2019. PMID: 30970355 Review. - Renal anemia syndromes in iraqi hemodialysis patients according to iron status.
Ali A, Salih RM. Ali A, et al. Saudi J Kidney Dis Transpl. 2018 Jan-Feb;29(1):127-135. doi: 10.4103/1319-2442.225182. Saudi J Kidney Dis Transpl. 2018. PMID: 29456218 - Update on Anemia in ESRD and Earlier Stages of CKD: Core Curriculum 2018.
Fishbane S, Spinowitz B. Fishbane S, et al. Am J Kidney Dis. 2018 Mar;71(3):423-435. doi: 10.1053/j.ajkd.2017.09.026. Epub 2018 Jan 11. Am J Kidney Dis. 2018. PMID: 29336855 Review. - Prevalence, correlates and outcomes of absolute and functional iron deficiency anemia in nondialysis-dependent chronic kidney disease.
Awan AA, Walther CP, Richardson PA, Shah M, Winkelmayer WC, Navaneethan SD. Awan AA, et al. Nephrol Dial Transplant. 2021 Jan 1;36(1):129-136. doi: 10.1093/ndt/gfz192. Nephrol Dial Transplant. 2021. PMID: 31641775
Cited by
- Iron deficiency in dogs suffering from atopic dermatitis.
Ramos CF, Doulidis PG, Polakova N, Burgener IA, Jensen-Jarolim E, Cimarelli G, Panakova L, Roth-Walter F. Ramos CF, et al. BMC Vet Res. 2024 Nov 6;20(1):506. doi: 10.1186/s12917-024-04350-y. BMC Vet Res. 2024. PMID: 39506866 Free PMC article. - Transition from acute kidney injury to chronic kidney disease in a long-term murine model of Shiga toxin-induced hemolytic-uremic syndrome.
Wegener J, Dennhardt S, Loeffler I, Coldewey SM. Wegener J, et al. Front Immunol. 2024 Oct 10;15:1469353. doi: 10.3389/fimmu.2024.1469353. eCollection 2024. Front Immunol. 2024. PMID: 39450175 Free PMC article. - Effectiveness of Lactoferrin in the Treatment of Anemia in Chronic Kidney Disease: A Single-Center Pilot Study.
Kekan K, Divyaveer S, Kashyap M, Premkumar M, Zohmangaihi D, Mallik N, Lad D, Sharma A, Shankar S G, Garg S, Prabhahar A, Chaudhary A, Suleiman S, Rather I, Verma M, Jassal RS, Kohli HS. Kekan K, et al. Indian J Nephrol. 2024 May-Jun;34(3):222-227. doi: 10.4103/ijn.ijn_13_23. Epub 2023 Oct 23. Indian J Nephrol. 2024. PMID: 39114392 Free PMC article. - Iron Deficiency Might Impair the Recovery of Left Ventricular Function after Surgical Revascularization in Diabetic Patients: A Retrospective Study.
Nan Y, Tiemuerniyazi X, Song Y, Chen L, Yang Z, Zhang S, Feng W. Nan Y, et al. Rev Cardiovasc Med. 2023 Jul 17;24(7):209. doi: 10.31083/j.rcm2407209. eCollection 2023 Jul. Rev Cardiovasc Med. 2023. PMID: 39077024 Free PMC article. - A whole-body mechanistic physiologically-based pharmacokinetic modeling of intravenous iron.
Fan X, Cao K, Wong RSM, Yan X. Fan X, et al. Drug Deliv Transl Res. 2024 Jul 24. doi: 10.1007/s13346-024-01675-x. Online ahead of print. Drug Deliv Transl Res. 2024. PMID: 39048784
References
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. .; CHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 - PubMed
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. .; TREAT Investigators: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 - PubMed
- Levin A, Djurdjev O, Thompson C, Barrett B, Ethier J, Carlisle E, et al. .: Canadian randomized trial of hemoglobin maintenance to prevent or delay left ventricular mass growth in patients with CKD. Am J Kidney Dis 46: 799–811, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical