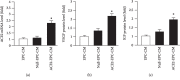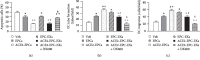Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) from Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell - PubMed (original) (raw)
Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) from Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell
Jinju Wang et al. Oxid Med Cell Longev. 2020.
Abstract
Angiotensin-converting enzyme 2 (ACE2) is an emerging cardiovascular protective target that mediates the metabolism of angiotensin (Ang) II into Ang (1-7). Our group has demonstrated that ACE2 overexpression enhances the function of endothelial progenitor cells (EPCs). Here, we investigated whether ACE2-primed EPCs (ACE2-EPCs) can protect cerebral microvascular endothelial cells (ECs) against injury and dysfunction in an in vitro model, with focusing on their exosomal and cytokine paracrine effects on endothelial mitochondria. Human EPCs were transfected with lentivirus containing null or human ACE2 cDNA (denoted as Null-EPCs and ACE2-EPCs, respectively). Their conditioned culture media, w/wo depletion of exosomes (ACE2-EPC-CMEX-, Null-EPC-CMEX-, ACE2-EPC-CM, and Null-EPC-CM), were used for coculture experiments. EC injury and dysfunction model was induced by Ang II before coculture. Apoptosis, angiogenic ability, mitochondrion functions (ROS production, membrane potential, fragmentation), and gene expressions (ACE2, Nox2, and Nox4) of ECs were analyzed. The supernatant was collected for measuring the levels of ACE2, Ang II/Ang-(1-7), and growth factors (VEGF and IGF). Our results showed that (1) ACE2-EPC-CM had higher levels of ACE2, Ang (1-7), VEGF, and IGF than that of Null-EPC-CM. (2) Ang II-injured ECs displayed an increase of apoptotic rate and reduction in tube formation and migration abilities, which were associated with ACE2 downregulation, Ang II/Ang (1-7) imbalance, Nox2/Nox4 upregulation, ROS overproduction, an increase of mitochondrion fragmentation, and a decrease of membrane potential. (3) ACE2-EPC-CM had better protective effects than Null-EPC-CM on Ang II-injured ECs, which were associated with the improvements on ACE2 expression, Ang II/Ang (1-7) balance, and mitochondrial functions. (4) ACE2-EPC-CMEX- and Null-EPC-CMEX- showed reduced effects as compared to ACE2-EPCs-CM and Null-EPCs-CM. In conclusion, our data demonstrate that ACE2 overexpression can enhance the protective effects of EPCs on ECs injury, majorly through the exosomal effects on mitochondrial function.
Copyright © 2020 Jinju Wang et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Figure 1
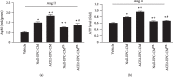
ACE2 transfection increased the mRNA level of ACE2 and the protein levels of VEGF and IGF. (a) qRT-PCR analysis of the ACE2 mRNA level in the three types of CM. (b, c) ELISA analysis of VEGF and IGF protein levels in the three types of CM. ∗P < 0.05 vs. EPC-CM; #P < 0.05 vs. Null-EPC-CM. N = 6/group.
Figure 2
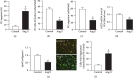
Characterization of Ang II-induced EC injury model. (a) EC apoptosis. (b, c) Tube formation and migration abilities. (d) ACE2 mRNA level in ECs. (e) Representative JC-1 staining images and summary data of _Δψ_M in ECs. _Δψ_M: mitochondrion membrane potential (MMP, red/green). Bar: 50 μ_m. (f) Summary data showing the percentage of cells with fragmented mitochondria. ∗_P < 0.05 vs. control. N = 6/group.
Figure 3
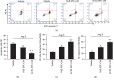
ACE2-EPC-CM decreased Ang II-induced EC apoptosis and dysfunction. (a) Representative flow plots of EC apoptosis. (b) Summarized data of EC apoptosis. (c) Tube formation. (d) Migration. ∗P < 0.05 vs. vehicle; +P < 0.05 vs. Null-EPC-CM. N = 6/group.
Figure 4
ACE2-EPC-CM upregulated ACE2 level and improved Ang II/Ang (1–7) balance on Ang II-induced ECs. (a) ACE2 mRNA level of ECs. (b) The ratio of Ang II/Ang (1–7). ∗P < 0.05 vs. vehicle; +P < 0.05 vs. Null-EPC-CM; #P < 0.05 vs. ACE2-EPC-CM. N = 6/group.
Figure 5
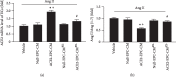
ACE2-EPC-CM increased MMP and ATP production. (a) The change of MMP in ECs after coincubation with different CM. (b) The ATP levels in ECs after different treatments. ∗P < 0.05 vs. vehicle; +P < 0.05 vs. Null-EPC-CM; #P < 0.05 vs. ACE2-EPC-CM. N = 6/group.
Figure 6
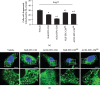
ACE2-EPC-CM decreased mitochondrion fragmentation. (a) Summary data showing the percentage of cells with fragmented mitochondria. (b) Representative confocal images showing the morphology of the mitochondrion. Enlarged images are magnifications of the mitochondria at the indicated area. Bar: 10 μ_m. ∗_P < 0.05 vs. vehicle; +P < 0.05 vs. Null-EPC-CM; #P < 0.05 vs. ACE2-EPC-CM. N = 6/group.
Figure 7
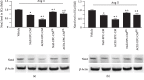
ACE2-EPC-CM decreased the expressions of Nox2 and Nox4. (a, b) Representative bands and summarized data showing the expressions of Nox2 and Nox4 in ECs exposed to Ang II. ∗P < 0.05 vs. vehicle; +P < 0.05 vs. Null-EPC-CM; #P < 0.05 vs. ACE2-EPC-CM. N = 6/group.
Figure 8
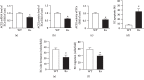
The dysfunction of ECs from R+ mice with decreased levels of ACE2 expression and activity. (a) ACE2 mRNA level of ECs. (b) ACE2 protein level of ECs. (c) ACE2 activity of ECs. (d) EC apoptotic rate is increased in R+ mice. (e, f) EC function is impaired in R+ mice. ∗P < 0.05 vs. vehicle; +P < 0.05 vs. WT. N = 10/group.
Figure 9
ACE2-EPC-EXs decreased apoptosis and improved the function of ECs from R+ mice. (a) The effects of ACE2-EPC-EXs on EC apoptosis. (b) The effects of ACE2-EPC-EXs on EC tube formation. (c) The effects of ACE2-EPC-EXs on EC migration. ∗P < 0.05 vs. Veh; +P < 0.05 vs. EPCs; #P < 0.05 vs. EPC-EXs; &P < 0.05 vs. ACE2-EPC-EXs. N = 10/group.
Similar articles
- ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway.
Zhang C, Wang J, Ma X, Wang W, Zhao B, Chen Y, Chen C, Bihl JC. Zhang C, et al. J Cell Mol Med. 2018 Mar;22(3):1873-1882. doi: 10.1111/jcmm.13471. Epub 2018 Jan 24. J Cell Mol Med. 2018. PMID: 29363860 Free PMC article. - Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy.
Chen J, Xiao X, Chen S, Zhang C, Chen J, Yi D, Shenoy V, Raizada MK, Zhao B, Chen Y. Chen J, et al. Hypertension. 2013 Mar;61(3):681-9. doi: 10.1161/HYPERTENSIONAHA.111.00202. Epub 2012 Dec 24. Hypertension. 2013. PMID: 23266545 Free PMC article. - Loading MiR-210 in Endothelial Progenitor Cells Derived Exosomes Boosts Their Beneficial Effects on Hypoxia/Reoxygeneation-Injured Human Endothelial Cells via Protecting Mitochondrial Function.
Ma X, Wang J, Li J, Ma C, Chen S, Lei W, Yang Y, Liu S, Bihl J, Chen C. Ma X, et al. Cell Physiol Biochem. 2018;46(2):664-675. doi: 10.1159/000488635. Epub 2018 Mar 29. Cell Physiol Biochem. 2018. PMID: 29621777 - ACE2 and the Homolog Collectrin in the Modulation of Nitric Oxide and Oxidative Stress in Blood Pressure Homeostasis and Vascular Injury.
Yang G, Chu PL, Rump LC, Le TH, Stegbauer J. Yang G, et al. Antioxid Redox Signal. 2017 Apr 20;26(12):645-659. doi: 10.1089/ars.2016.6950. Epub 2017 Jan 12. Antioxid Redox Signal. 2017. PMID: 27889958 Review. - ACE2 of the heart: From angiotensin I to angiotensin (1-7).
Keidar S, Kaplan M, Gamliel-Lazarovich A. Keidar S, et al. Cardiovasc Res. 2007 Feb 1;73(3):463-9. doi: 10.1016/j.cardiores.2006.09.006. Epub 2006 Sep 19. Cardiovasc Res. 2007. PMID: 17049503 Review.
Cited by
- SARS-CoV-2 spike protein enhances MAP4K3/GLK-induced ACE2 stability in COVID-19.
Chuang HC, Hsueh CH, Hsu PM, Huang RH, Tsai CY, Chung NH, Chow YH, Tan TH. Chuang HC, et al. EMBO Mol Med. 2022 Sep 7;14(9):e15904. doi: 10.15252/emmm.202215904. Epub 2022 Jul 27. EMBO Mol Med. 2022. PMID: 35894122 Free PMC article. - COVID-19 Related Coagulopathy: A Distinct Entity?
Marchandot B, Sattler L, Jesel L, Matsushita K, Schini-Kerth V, Grunebaum L, Morel O. Marchandot B, et al. J Clin Med. 2020 May 31;9(6):1651. doi: 10.3390/jcm9061651. J Clin Med. 2020. PMID: 32486469 Free PMC article. Review. - A Role for Extracellular Vesicles in SARS-CoV-2 Therapeutics and Prevention.
Machhi J, Shahjin F, Das S, Patel M, Abdelmoaty MM, Cohen JD, Singh PA, Baldi A, Bajwa N, Kumar R, Vora LK, Patel TA, Oleynikov MD, Soni D, Yeapuri P, Mukadam I, Chakraborty R, Saksena CG, Herskovitz J, Hasan M, Oupicky D, Das S, Donnelly RF, Hettie KS, Chang L, Gendelman HE, Kevadiya BD. Machhi J, et al. J Neuroimmune Pharmacol. 2021 Jun;16(2):270-288. doi: 10.1007/s11481-020-09981-0. Epub 2021 Feb 5. J Neuroimmune Pharmacol. 2021. PMID: 33544324 Free PMC article. Review. - Impact of the Renin-Angiotensin System on the Endothelium in Vascular Dementia: Unresolved Issues and Future Perspectives.
Noureddine FY, Altara R, Fan F, Yabluchanskiy A, Booz GW, Zouein FA. Noureddine FY, et al. Int J Mol Sci. 2020 Jun 16;21(12):4268. doi: 10.3390/ijms21124268. Int J Mol Sci. 2020. PMID: 32560034 Free PMC article. Review. - The collectrin-like part of the SARS-CoV-1 and -2 receptor ACE2 is shed by the metalloproteinases ADAM10 and ADAM17.
Niehues RV, Wozniak J, Wiersch F, Lilienthal E, Tacken N, Schumertl T, Garbers C, Ludwig A, Düsterhöft S. Niehues RV, et al. FASEB J. 2022 Mar;36(3):e22234. doi: 10.1096/fj.202101521R. FASEB J. 2022. PMID: 35199397 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous