In Vitro Anti-HSV-1 Activity of Polyphenol-Rich Extracts and Pure Polyphenol Compounds Derived from Pistachios Kernels (Pistacia vera L.) - PubMed (original) (raw)
In Vitro Anti-HSV-1 Activity of Polyphenol-Rich Extracts and Pure Polyphenol Compounds Derived from Pistachios Kernels (Pistacia vera L.)
Maria Musarra-Pizzo et al. Plants (Basel). 2020.
Abstract
Natural compounds are a prominent source of novel antiviral drugs. Several reports have previously shown the antimicrobial activity of pistachio polyphenol extracts. Therefore, the aim of our research was to investigate the activity of polyphenol-rich extracts of natural shelled (NPRE) pistachios kernels (Pistacia vera L.) on herpes simplex virus type 1 (HSV-1) replication. The Vero cell line was used to assess the cytotoxicity and antiviral activity. The cell viability was calculated by detection of cellular ATP after treatment with various concentrations of NPRE. For antiviral studies, five nontoxic-concentrations (0.1, 0.2, 0.4, 0.6, 0.8 mg/mL) were tested. Our study demonstrated that treatment with NPRE (0.4, 0.6, 0.8 mg/mL) reduced the expression of the viral proteins ICP8 (infected cell polypeptide 8), UL42 (unique long UL42 DNA polymerase processivity factor) , and US11 (unique short US11 protein), and resulted in a decrease of viral DNA synthesis. The 50% cytotoxic concentration (CC50), 50% inhibitory concentration (EC50), and the selectivity index (SI) values for NPRE were 1.2 mg/mL, 0.4mg/mL, and 3, respectively. Furthermore, we assessed the anti-herpetic effect of a mix of pure polyphenol compounds (NS MIX) present in NPRE. In conclusion, our findings indicate that natural shelled pistachio kernels have remarkable inhibitory activity against HSV-1.
Keywords: antiviral; herpes simplex virus; pistachios; polyphenols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
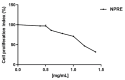
Figure 1
Cell viability of Vero cells treated with increasing concentration of polyphenol-rich extracts from natural shelled pistachios kernels (NPRE). The cell proliferation index (%) was calculated by means of cellular ATP level measured after 72 h treatment. The values were expressed as percentages of treated vs. control cells (DMSO). Each value is the mean ± standard deviation (SD) of three experiments.
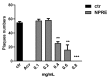
Figure 2
Effects of NPRE on herpes simplex 1 (HSV-1) replication by plaque reduction assay. Vero cells were infected with HSV-1 (50 plaque forming units (PFU)/100 µL) for 1 h and then treated with NPRE (0.1, 0.2, 0.4, 0.6, 0.8 mg/mL). The DMSO was used in the control samples and acyclovir (20 µM) was used as positive control. Data are expressed as a mean (± SD) of at least three experiments, and asterisks (**, and ***) indicate the significance of _p_-values less than 0.01 and 0.001, respectively.
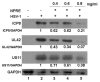
Figure 3
Analysis of viral protein expression by Western blot analysis.
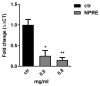
Figure 4
Relative quantization of viral DNA with specific HSV-1 TaqMan probe in real-time PCR. DMSO was used in the infected control samples. Data are expressed as a mean (± SD) of at least three experiments and asterisks (* and **) indicate the significance of _p_-values less than 0.05 and 0.01, respectively.
Figure 5
Effect of the NP MIX treatment on HSV-1 replication. Vero cells were either infected or mock-infected with HSV-1-VP26 GFP (green fluorescent protein-tagged capsid protein VP26) at multiplicity of infection (MOI) 1, as described in the Materials and Methods section. Then, the cells were analysed at 24 h post infection (p.i.): (a) fluorescent images showed the green dots representing the VP26 GFP viral antigen localization (I)—Hoechst (blue) was used to stain the nuclei (II) and the merged images are shown in column III; (b) the graph is indicative of the percentage of VP26 GFP-positive cells; (c) Western blot analysis of VP26 GFP-tagged protein. Data are expressed as a mean (± SD) of at least three experiments, and asterisks (***) indicate the significance of _p_-values less than 0.001.
Similar articles
- Mechanistic Understanding of the Antiviral Properties of Pistachios and Zeaxanthin against HSV-1.
Pennisi R, Trischitta P, Tamburello MP, Barreca D, Mandalari G, Sciortino MT. Pennisi R, et al. Viruses. 2023 Jul 29;15(8):1651. doi: 10.3390/v15081651. Viruses. 2023. PMID: 37631995 Free PMC article. - Biochemical Characterization of Clinical Strains of Staphylococcus spp. and Their Sensitivity to Polyphenols-Rich Extracts from Pistachio (Pistacia vera L.).
La Camera E, Bisignano C, Crisafi G, Smeriglio A, Denaro M, Trombetta D, Mandalari G. La Camera E, et al. Pathogens. 2018 Oct 22;7(4):82. doi: 10.3390/pathogens7040082. Pathogens. 2018. PMID: 30360375 Free PMC article. - Pistacia vera L. as natural source against antimicrobial and antiviral resistance.
Mandalari G, Pennisi R, Gervasi T, Sciortino MT. Mandalari G, et al. Front Microbiol. 2024 Jul 1;15:1396514. doi: 10.3389/fmicb.2024.1396514. eCollection 2024. Front Microbiol. 2024. PMID: 39011148 Free PMC article. Review. - In vitro antimicrobial activity of pistachio (Pistacia vera L.) polyphenols.
Bisignano C, Filocamo A, Faulks RM, Mandalari G. Bisignano C, et al. FEMS Microbiol Lett. 2013 Apr;341(1):62-7. doi: 10.1111/1574-6968.12091. Epub 2013 Feb 11. FEMS Microbiol Lett. 2013. PMID: 23350629 - Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects.
Mandalari G, Barreca D, Gervasi T, Roussell MA, Klein B, Feeney MJ, Carughi A. Mandalari G, et al. Plants (Basel). 2021 Dec 22;11(1):18. doi: 10.3390/plants11010018. Plants (Basel). 2021. PMID: 35009022 Free PMC article. Review.
Cited by
- Antiviral Activity Exerted by Natural Products against Human Viruses.
Musarra-Pizzo M, Pennisi R, Ben-Amor I, Mandalari G, Sciortino MT. Musarra-Pizzo M, et al. Viruses. 2021 May 4;13(5):828. doi: 10.3390/v13050828. Viruses. 2021. PMID: 34064347 Free PMC article. Review. - Redox-Modulating Agents in the Treatment of Viral Infections.
Checconi P, De Angelis M, Marcocci ME, Fraternale A, Magnani M, Palamara AT, Nencioni L. Checconi P, et al. Int J Mol Sci. 2020 Jun 8;21(11):4084. doi: 10.3390/ijms21114084. Int J Mol Sci. 2020. PMID: 32521619 Free PMC article. Review. - Phytochemical Characterization of Olea europea Leaf Extracts and Assessment of Their Anti-Microbial and Anti-HSV-1 Activity.
Ben-Amor I, Musarra-Pizzo M, Smeriglio A, D'Arrigo M, Pennisi R, Attia H, Gargouri B, Trombetta D, Mandalari G, Sciortino MT. Ben-Amor I, et al. Viruses. 2021 Jun 7;13(6):1085. doi: 10.3390/v13061085. Viruses. 2021. PMID: 34200316 Free PMC article. - In Vitro Anti-Epstein Barr Virus Activity of Olea europaea L. Leaf Extracts.
Ben-Amor I, Gargouri B, Attia H, Tlili K, Kallel I, Musarra-Pizzo M, Sciortino MT, Pennisi R. Ben-Amor I, et al. Plants (Basel). 2021 Nov 12;10(11):2445. doi: 10.3390/plants10112445. Plants (Basel). 2021. PMID: 34834807 Free PMC article. - Mechanisms of Plant Antioxidants Action.
Barreca D. Barreca D. Plants (Basel). 2020 Dec 25;10(1):35. doi: 10.3390/plants10010035. Plants (Basel). 2020. PMID: 33375600 Free PMC article.
References
- Tyler K.L. Herpes simplex virus infections of the central nervous system: Encephalitis and meningitis, including Mollaret’s. Herpes. 2004;11(Suppl. 2):57A–64A. - PubMed
- Whitley R. New approaches to the therapy of HSV infections. Herpes. 2006;13:53–55. - PubMed
- Chakrabarti S., Pillay D., Ratcliffe D., Cane P.A., Cllinghan K.E., Milligan D.W. Resistance to antiviral drugs in herpes simplex virus infections among allogenic stem cell transplant recipients: Risk factors and prognostic significance. J. Infect. Dis. 2000;181:2055–2058. doi: 10.1086/315524. - DOI - PubMed
- Chen Y., Scieux V., Garrait V., Socie G., Rocha V., Molina J.M., Thouvenot D., Morfin F., Hocqueloux I., Garderei L., et al. Resistant herpes simplex virus type 1 infection: An emerging concern after allogenetic stem cell transplantation. Clin. Infect. Dis. 2000;31:927–935. doi: 10.1086/314052. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous