The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus - PubMed (original) (raw)
The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus
Calvin J Gordon et al. J Biol Chem. 2020.
Abstract
Antiviral drugs for managing infections with human coronaviruses are not yet approved, posing a serious challenge to current global efforts aimed at containing the outbreak of severe acute respiratory syndrome-coronavirus 2 (CoV-2). Remdesivir (RDV) is an investigational compound with a broad spectrum of antiviral activities against RNA viruses, including severe acute respiratory syndrome-CoV and Middle East respiratory syndrome (MERS-CoV). RDV is a nucleotide analog inhibitor of RNA-dependent RNA polymerases (RdRps). Here, we co-expressed the MERS-CoV nonstructural proteins nsp5, nsp7, nsp8, and nsp12 (RdRp) in insect cells as a part a polyprotein to study the mechanism of inhibition of MERS-CoV RdRp by RDV. We initially demonstrated that nsp8 and nsp12 form an active complex. The triphosphate form of the inhibitor (RDV-TP) competes with its natural counterpart ATP. Of note, the selectivity value for RDV-TP obtained here with a steady-state approach suggests that it is more efficiently incorporated than ATP and two other nucleotide analogs. Once incorporated at position i, the inhibitor caused RNA synthesis arrest at position i + 3. Hence, the likely mechanism of action is delayed RNA chain termination. The additional three nucleotides may protect the inhibitor from excision by the viral 3'-5' exonuclease activity. Together, these results help to explain the high potency of RDV against RNA viruses in cell-based assays.
Keywords: Ebola virus (EBOV); Middle East respiratory syndrome coronavirus (MERS–CoV); RNA chain termination; RNA-dependent RNA polymerase (RdRp); SARS–CoV-2; antiviral drug; coronavirus; drug development; enzyme inhibitor; nucleoside/nucleotide analog; plus-stranded RNA virus; positive-sense RNA virus; remdesivir; viral polymerase; viral replicase.
© 2020 Gordon et al.
Conflict of interest statement
M. G. has previously received funding from Gilead Sciences in support for the study of EBOV RdRp inhibition by RDV
Figures
Figure 1.
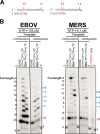
Expression, purification, and characterization of the MERS RdRp complex. A, the construct contains nonstructural proteins nsp5, nsp7, nsp8, and nsp12. Red rectangles indicate original nsp5 protease cleavage sites. His8 and Strep indicate the locations of histidine and strep tags, respectively. B, a snapshot of a sequence alignment (T-Coffee) of representative RdRp enzymes from positive-sense RNA genome viruses illustrating sequence conservation within RdRp motif C.C, SDS-PAGE migration pattern of the purified enzyme preparations stained with Coomassie Brilliant Blue G-250 dye. Proteins migrating at ∼100 and ∼25 kDa contain nsp12 and nsp8, respectively. D, RNA synthesis on a short model primer/template substrate. Template and primer were both phosphorylated (p) at their 5′-ends. _G_indicates incorporation of the radiolabeled nucleotide opposite template position 5. RNA synthesis was monitored with the purified MERS RdRp complex wt (motif C = SDD) and active-site mutant (motif C = SNN) in the presence of NTP combinations designed to generate specific products. Lanes m illustrate the migration pattern of the radiolabeled 4-nucleotide-long primer. HCV, hepatitis C virus;NoV, norovirus; PoV, poliovirus.
Figure 2.
Patterns of inhibition of RNA synthesis with RDV-TP. A, RNA primer/template substrates used to test multiple (left panel) or single (right panel) incorporations of RDV-TP.G indicates incorporation of the radiolabeled nucleotide opposite template position 5. i indicates incorporation site for the first (left panel) or the only (right panel) RDV-TP.Full length indicates the full-template length products of RNA synthesis. B, RDV-TP incorporation was monitored with purified EBOV and MERS RdRp complexes in the presence of the indicated combinations of NTPs and RDV-TP.
Figure 3.
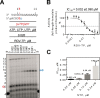
Competition between RDV-TP and ATP. A, the RNA primer/template substrate used in this assay is shown_above_ the gel. G indicates incorporation of the radiolabeled nucleotide opposite template position 5. Position _i_allows incorporation of ATP or RDV-TP. RNA synthesis was monitored with purified MERS RdRp complex in the presence of 0.02 μ
m
ATP, CTP, and UTP mix and increasing concentrations of RDV-TP as indicated. B, graphic representation and IC50 determination fitting of quantified data from_A_. The error bars represent standard deviation of the data within four independent experiments. C, graphic representation of the relationship between IC50 values for RDV-TP measured at different NTP concentrations. The average IC50 values for RDV-TP are shown above the corresponding bars. The_error bars_ represent standard deviation of the data within at least three independent experiments.
Figure 4.
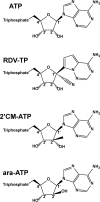
Chemical structures of ATP and ATP analogs.
Similar articles
- Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency.
Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Götte M. Gordon CJ, et al. J Biol Chem. 2020 May 15;295(20):6785-6797. doi: 10.1074/jbc.RA120.013679. Epub 2020 Apr 13. J Biol Chem. 2020. PMID: 32284326 Free PMC article. - Efficient incorporation and template-dependent polymerase inhibition are major determinants for the broad-spectrum antiviral activity of remdesivir.
Gordon CJ, Lee HW, Tchesnokov EP, Perry JK, Feng JY, Bilello JP, Porter DP, Götte M. Gordon CJ, et al. J Biol Chem. 2022 Feb;298(2):101529. doi: 10.1016/j.jbc.2021.101529. Epub 2021 Dec 23. J Biol Chem. 2022. PMID: 34953856 Free PMC article. - Template-dependent inhibition of coronavirus RNA-dependent RNA polymerase by remdesivir reveals a second mechanism of action.
Tchesnokov EP, Gordon CJ, Woolner E, Kocinkova D, Perry JK, Feng JY, Porter DP, Götte M. Tchesnokov EP, et al. J Biol Chem. 2020 Nov 20;295(47):16156-16165. doi: 10.1074/jbc.AC120.015720. Epub 2020 Sep 23. J Biol Chem. 2020. PMID: 32967965 Free PMC article. - RNA-dependent RNA polymerase: Structure, mechanism, and drug discovery for COVID-19.
Jiang Y, Yin W, Xu HE. Jiang Y, et al. Biochem Biophys Res Commun. 2021 Jan 29;538:47-53. doi: 10.1016/j.bbrc.2020.08.116. Epub 2020 Sep 4. Biochem Biophys Res Commun. 2021. PMID: 32943188 Free PMC article. Review. - RNA-Dependent RNA Polymerase as a Target for COVID-19 Drug Discovery.
Zhu W, Chen CZ, Gorshkov K, Xu M, Lo DC, Zheng W. Zhu W, et al. SLAS Discov. 2020 Dec;25(10):1141-1151. doi: 10.1177/2472555220942123. Epub 2020 Jul 13. SLAS Discov. 2020. PMID: 32660307 Free PMC article. Review.
Cited by
- Advancements in the Development of Anti-SARS-CoV-2 Therapeutics.
Huang J, Ma Q, Su Z, Cheng X. Huang J, et al. Int J Mol Sci. 2024 Oct 9;25(19):10820. doi: 10.3390/ijms251910820. Int J Mol Sci. 2024. PMID: 39409149 Free PMC article. Review. - Binding Mechanism of the Active Form of Molnupiravir to RdRp of SARS-CoV-2 and Designing Potential Analogues: Insights from Molecular Dynamics Simulations.
Carbone J, Paradis NJ, Brunt D, Wu C. Carbone J, et al. ACS Omega. 2024 Sep 24;9(40):41583-41598. doi: 10.1021/acsomega.4c05469. eCollection 2024 Oct 8. ACS Omega. 2024. PMID: 39398139 Free PMC article. - Among Patients with COVID-19, should Remdesivir be Used for Treatment? A Systematic Review and Meta-analysis.
Tan-Lim CSC, Esteban-Ipac NAR. Tan-Lim CSC, et al. Acta Med Philipp. 2024 Aug 15;58(14):50-66. doi: 10.47895/amp.vi0.7288. eCollection 2024. Acta Med Philipp. 2024. PMID: 39238554 Free PMC article. - An in vitro study for reducing the cytotoxicity and dose dumping risk of remdesivir via entrapment in nanostructured lipid carriers.
Amiri F, Ziaei Chamgordani S, Ghourchian H. Amiri F, et al. Sci Rep. 2024 Aug 21;14(1):19360. doi: 10.1038/s41598-024-70003-7. Sci Rep. 2024. PMID: 39169059 Free PMC article. - Viral replication organelles: the highly complex and programmed replication machinery.
Deng H, Cao H, Wang Y, Li J, Dai J, Li LF, Qiu HJ, Li S. Deng H, et al. Front Microbiol. 2024 Jul 31;15:1450060. doi: 10.3389/fmicb.2024.1450060. eCollection 2024. Front Microbiol. 2024. PMID: 39144209 Free PMC article. Review.
References
- Sheahan T. P., Sims A. C., Leist S. R., Schäfer A., Won J., Brown A. J., Montgomery S. A., Hogg A., Babusis D., Clarke M. O., Spahn J. E., Bauer L., Sellers S., Porter D., Feng J. Y., et al. (2020) Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon β against MERS–CoV. Nat. Commun. 11, 222 10.1038/s41467-019-13940-6 - DOI - PMC - PubMed
- Siegel D., Hui H. C., Doerffler E., Clarke M. O., Chun K., Zhang L., Neville S., Carra E., Lew W., Ross B., Wang Q., Wolfe L., Jordan R., Soloveva V., Knox J., et al. . (2017) Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J. Med. Chem. 60, 1648–1661 10.1021/acs.jmedchem.6b01594 - DOI - PubMed
- Lo M. K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A. L., Flint M., McMullan L. K., Siegel D., Clarke M. O., Mackman R. L., Hui H. C., Perron M., Ray A. S., Cihlar T., et al. (2017) GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses. Sci. Rep. 7, 43395 10.1038/srep43395 - DOI - PMC - PubMed
- Warren T. K., Jordan R., Lo M. K., Ray A. S., Mackman R. L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H. C., Larson N., Strickley R., Wells J., Stuthman K. S., Van Tongeren S. A., et al. . (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531, 381–385 10.1038/nature17180 - DOI - PMC - PubMed
- Agostini M. L., Andres E. L., Sims A. C., Graham R. L., Sheahan T. P., Lu X., Smith E. C., Case J. B., Feng J. Y., Jordan R., Ray A. S., Cihlar T., Siegel D., Mackman R. L., Clarke M. O., et al. . (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 9, e00221–18 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous