Highly regulated, diversifying NTP-dependent biological conflict systems with implications for the emergence of multicellularity - PubMed (original) (raw)
Highly regulated, diversifying NTP-dependent biological conflict systems with implications for the emergence of multicellularity
Gurmeet Kaur et al. Elife. 2020.
Abstract
Social cellular aggregation or multicellular organization pose increased risk of transmission of infections through the system upon infection of a single cell. The generality of the evolutionary responses to this outside of Metazoa remains unclear. We report the discovery of several thematically unified, remarkable biological conflict systems preponderantly present in multicellular prokaryotes. These combine thresholding mechanisms utilizing NTPase chaperones (the MoxR-vWA couple), GTPases and proteolytic cascades with hypervariable effectors, which vary either by using a reverse transcriptase-dependent diversity-generating system or through a system of acquisition of diverse protein modules, typically in inactive form, from various cellular subsystems. Conciliant lines of evidence indicate their deployment against invasive entities, like viruses, to limit their spread in multicellular/social contexts via physical containment, dominant-negative interactions or apoptosis. These findings argue for both a similar operational 'grammar' and shared protein domains in the sensing and limiting of infections during the multiple emergences of multicellularity.
Keywords: AAA+ ATPase; DEATH domain; GTPase; apoptosis; chaperones; genetics; genomics; infectious disease; microbiology; multicellular prokaryotes.
Plain language summary
Bacteria are the most numerous lifeforms on the planet. Most bacteria live as single cells that grow and multiply independently within larger communities of microbes. However, some bacterial cells assemble to form more complex structures where individual cells might perform distinct roles. Such bacteria are referred to as ‘multicellular bacteria’. For example, cells of bacteria known as Streptomyces collectively form filaments that help the bacteria collect nutrients from their food sources, and aerial structures bearing reproductive spores. Bacteria in these filaments may come into contact with many other microbes in their surroundings including other bacteria within the same filament, other species of bacteria, and viruses. These contacts often lead to conflict, for example, if the microbes compete with each other for nutrients or if a virus tries to attack the bacteria. Bacteria have evolved immune systems that detect other microbes and use antibiotics, toxins and other defense mechanisms against them. Compared to single-celled bacteria, multicellular bacteria may be more vulnerable to threats from viruses because once a virus has overcome the defenses of one cell in the multicellular assembly, it may be easier for it to kill, or spread to the other cells. However, it is not clear how these systems evolved to deal with the unique problems of multicellular bacteria. Now, Kaur, Burroughs et al. have used computational approaches to search for new immune systems in diverse multicellular bacteria. The new classes of systems they found are each made of different molecular components, but all require a large input of energy to be activated. This activation barrier prevents the bacterial cells from deploying weapons unless the signal from the enemy microbe crosses a high enough threshold. Many tools used in molecular biology, and increasingly in medicine, have been derived from the immune systems of bacteria, such as the enzymes that cut or edit DNA. The findings of Kaur, Burroughs et al. may aid the development of new tools that specifically bind to viruses or other dangerous microbes, or inhibit their ability to interact with components in cells. The next step would be to perform experiments using some of the immune systems identified in this work.
Conflict of interest statement
GK, AB, LI, LA No competing interests declared
Figures
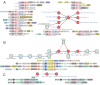
Figure 1.. Identification of novel conflict systems and overview of core ternary system components.
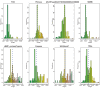
(A) Flowchart showing the process used to identify the conflict systems in this study. (B–C) Topology diagrams of the vWA (B) and MoxR (C) domains highlighting characteristic conserved features. (D) Multiple sequence alignment of VMAP-C domain. Sequences are labeled to the left by organism abbreviation (see Figure 1—source data 1) and accession number. Predicted secondary structure is shown on top and the consensus conservation used for coloring is given below. (E) Boxplot comparing sequence entropy values for VMAP core ternary system domains, collected from a common set of genomes containing all four components. Significant p-values determined by Wilcoxon Rank-Sum Test: *, p-value<2.2e-16. (F) Distribution of the number of effector domains C-terminally fused to the core vWA domain of the classical ternary systems. Dashed vertical lines, colored in cyan and blue, indicate median and mean domain number, respectively. (G) Histogram of the length distribution of the core vWA domain-containing component of the VMAP ternary systems, with dashed line as in (F).
Figure 1—figure supplement 1.. Histograms of vWA-fused effector protein length distributions by effector domain type.
Dashed blue vertical line indicates mean length.
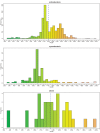
Figure 1—figure supplement 2.. Histograms of vWA-fused effector protein length distributions by taxonomy.
Dashed blue vertical line indicates mean length.
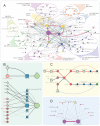
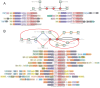
Figure 2.. Emergence of multicellularity across prokaryotes and the presence of the newly-identified conflict systems.
Tree of prokaryotic life (left) with general description and illustrations of known multicellular arrangements within clades (center) juxtaposed against presence/absence of the described systems (right). Coloring of clade labels matches multicellular descriptions. On the right, the fraction of organisms containing a system within a specific clade from the curated database (Materials and methods) is color-coded according to bottom legend. The first column on the right provides the fraction of known multicellular organisms within a clade in the curated database.
Figure 2—figure supplement 1.. Hypergeometric distributions of the expected and observed number of multicellular prokaryotic organisms in each system type.
System type is labeled above each distribution, with p-value and total number of systems identified (n = sum of multicellular and non-multicellular systems). Dashed vertical red lines are plotted at the number of observed systems found in multicellular organisms.
Figure 3.. Diversity of classical ternary systems.
(A) Evolution of the core components of the classical ternary systems across a common set of genomes. Scope of domain architectural diversity for the genomes under consideration are provided below for the vWA and VMAP-C components, linked to their respective trees by numbering (vWA) or color-coding (VMAP-C). (B) Generalized contextual diagram of the core components of the VMAP ternary system. The basic components are shown as nodes connected by arrows based on their gene neighborhood organization. The arrowhead points to the component encoded in the 3’ direction on the genome. Core conserved components are shown as red circles. Example gene neighborhoods are provided, linked to distinct subtypes of the ternary system by background coloring. Poorly-conserved genes appearing in a neighborhood are unlabeled and depicted in white boxes. Accession numbers and organism names provided as labels below each neighborhood.
Figure 4.. vWA architectural network and generalized classical ternary system diagrams.
(A) vWA domain architecture network of the MoxR-vWA-centric ternary systems. Domains linked in the same polypeptide are connected by arrows with the arrowhead pointing to the C-terminal domain. Node size is scaled based on the relative frequency of occurrence of the domain in the systems and edge thickness is scaled based on the relative frequency of edge occurrence. Edges with >148 occurrences are colored maroon, whereas those with >14 connections are shown in cadet blue, others are shown in grey. Functionally similar domains are identically colored as follows: blue, nucleotide-dependent signaling; purple, adaptor, superstructure-forming, and structural; dark red, peptidase; dark-green, DNA-targeting; light blue, cell division-related; yellow, apoptosis-related; orange, RNA-targeting; light green, macromolecule modification; red, miscellaneous effector. Further these have been grouped together to the extent possible and indicated on the network. (B) Generalized contextual diagram of VMAP architecture, as described in Figure 3B. (C) Detailed contextual diagram of the MoxR-vWA-centric ternary systems, depicting mutual exclusivity of components of peptide-modification accessory systems and the Trypco-trypsin and α/β hydrolase-CASPASE peptidase pairings. See 2B for convention. Arrow colors reflect the distinctness of the contexts. (D) FGS domain architectural subnetwork of the VMAP ternary systems depicting its diverse domain associations.
Figure 5.. Generalized contextual diagrams and example genome contexts of other described ternary systems.
(A–C) Generalized contextual diagrams for the components of the (A) iSTAND-, (B) FtsH-, and (C) β-propeller-containing systems. Coloring and connectivity as described in previous legends. Dotted lines reflect connections that are not universally present in a contextual theme. Example gene neighborhoods provided for systems as described in legend to Figure 3B.
Figure 6.. Generalized contextual diagrams and example genome contexts for the DO-GTPase systems.
(A–B) Generalized contextual diagrams for the components of the DO-GTPase (A) GAP1-N1 and (B) GAP1-N2 systems. Coloring and connectivity as described in previous legends. Arrow colors reflect distinct contextual themes. Black arrows reflect connections present in more than one contextual theme. Example gene neighborhoods provided for systems as described in legend to Figure 3B.
Figure 7.. Example gene neighborhoods.
(A) Illustrating the EAD-coupling principle, (B) of the NucA-peptidase system and (C) of the EACC1 two-gene system. Coloring and design as described in Figure 3B. EACC1 domain in (C) is colored in orange across systems, underscoring the conservation of the EACC1 component relative to the diversity of the associated CASPASE domain-fused component.
Figure 8.. Systems categorization and connections to eukaryotic apoptotic systems.
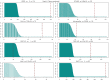
(A) Schematic representation and categorization of the systems described here placed in the context of other categories of biological conflict system classes. The various components of the systems described in this manuscript are depicted in their respective boxes along with a plausible mechanism of action, involving invader sensing, activation, proteolysis and effector deployment. The ternary core of each sub-system is delineated. (B) Cross-superkingdom comparison of the shared ‘grammar’ and domain content across the systems described herein (left column) and those experimentally-characterized in eukaryotic apoptotic pathways (right column). (C) Multiple sequence alignment of newly-identified bacterial Death-like domain families. Top four sequences are of known animal Death superfamily structures. (D) Histograms situating the VMAP ternary systems in actinobacterial genomes according to location of vWA domain-containing gene by stop nucleotide position relative to entire normalized nucleotide length of the genome (left) and protein ordering relative to normalized total protein count in the genome (right). Displayed p-values result from comparison to distribution expected by random genome distribution, using the χ2 test.
Similar articles
- CoCoNuTs are a diverse subclass of Type IV restriction systems predicted to target RNA.
Bell RT, Sahakyan H, Makarova KS, Wolf YI, Koonin EV. Bell RT, et al. Elife. 2024 May 13;13:RP94800. doi: 10.7554/eLife.94800. Elife. 2024. PMID: 38739430 Free PMC article. - Bacterial death and TRADD-N domains help define novel apoptosis and immunity mechanisms shared by prokaryotes and metazoans.
Kaur G, Iyer LM, Burroughs AM, Aravind L. Kaur G, et al. Elife. 2021 Jun 1;10:e70394. doi: 10.7554/eLife.70394. Elife. 2021. PMID: 34061031 Free PMC article. - Viral diversity threshold for adaptive immunity in prokaryotes.
Weinberger AD, Wolf YI, Lobkovsky AE, Gilmore MS, Koonin EV. Weinberger AD, et al. mBio. 2012 Dec 4;3(6):e00456-12. doi: 10.1128/mBio.00456-12. mBio. 2012. PMID: 23221803 Free PMC article. - The molecular chaperone system and other anti-stress mechanisms in archaea.
Macario AJ, Conway De Macario E. Macario AJ, et al. Front Biosci. 2001 Feb 1;6:D262-83. doi: 10.2741/macario. Front Biosci. 2001. PMID: 11171552 Review. - Engineering Aspects of Olfaction.
Persaud KC. Persaud KC. In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 1. In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 1. PMID: 26042329 Free Books & Documents. Review.
Cited by
- Comprehensive classification of ABC ATPases and their functional radiation in nucleoprotein dynamics and biological conflict systems.
Krishnan A, Burroughs AM, Iyer LM, Aravind L. Krishnan A, et al. Nucleic Acids Res. 2020 Oct 9;48(18):10045-10075. doi: 10.1093/nar/gkaa726. Nucleic Acids Res. 2020. PMID: 32894288 Free PMC article. Review. - Conservation and similarity of bacterial and eukaryotic innate immunity.
Ledvina HE, Whiteley AT. Ledvina HE, et al. Nat Rev Microbiol. 2024 Jul;22(7):420-434. doi: 10.1038/s41579-024-01017-1. Epub 2024 Feb 28. Nat Rev Microbiol. 2024. PMID: 38418927 Free PMC article. Review. - Thousands of previously unknown phages discovered in whole-community human gut metagenomes.
Benler S, Yutin N, Antipov D, Rayko M, Shmakov S, Gussow AB, Pevzner P, Koonin EV. Benler S, et al. Microbiome. 2021 Mar 29;9(1):78. doi: 10.1186/s40168-021-01017-w. Microbiome. 2021. PMID: 33781338 Free PMC article. - CoCoNuTs are a diverse subclass of Type IV restriction systems predicted to target RNA.
Bell RT, Sahakyan H, Makarova KS, Wolf YI, Koonin EV. Bell RT, et al. Elife. 2024 May 13;13:RP94800. doi: 10.7554/eLife.94800. Elife. 2024. PMID: 38739430 Free PMC article. - Reappraisal of the DNA phosphorothioate modification machinery: uncovering neglected functional modalities and identification of new counter-invader defense systems.
Rakesh S, Aravind L, Krishnan A. Rakesh S, et al. Nucleic Acids Res. 2024 Feb 9;52(3):1005-1026. doi: 10.1093/nar/gkad1213. Nucleic Acids Res. 2024. PMID: 38163645 Free PMC article.
References
- Alayyoubi M, Guo H, Dey S, Golnazarian T, Brooks GA, Rong A, Miller JF, Ghosh P. Structure of the essential diversity-generating retroelement protein bAvd and its functionally important interaction with reverse transcriptase. Structure. 2013;21:266–276. doi: 10.1016/j.str.2012.11.016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
LinkOut - more resources
Full Text Sources