Mouse Heterochromatin Adopts Digital Compaction States without Showing Hallmarks of HP1-Driven Liquid-Liquid Phase Separation - PubMed (original) (raw)
. 2020 Apr 16;78(2):236-249.e7.
doi: 10.1016/j.molcel.2020.02.005. Epub 2020 Feb 25.
Anne Rademacher 2, Rifka Vlijm 3, Jana Tünnermann 2, Lukas Frank 2, Robin Weinmann 2, Elisabeth Schweigert 2, Klaus Yserentant 4, Johan Hummert 4, Caroline Bauer 2, Sabrina Schumacher 2, Ahmad Al Alwash 2, Christophe Normand 5, Dirk-Peter Herten 6, Johann Engelhardt 3, Karsten Rippe 7
Affiliations
- PMID: 32101700
- PMCID: PMC7163299
- DOI: 10.1016/j.molcel.2020.02.005
Mouse Heterochromatin Adopts Digital Compaction States without Showing Hallmarks of HP1-Driven Liquid-Liquid Phase Separation
Fabian Erdel et al. Mol Cell. 2020.
Abstract
The formation of silenced and condensed heterochromatin foci involves enrichment of heterochromatin protein 1 (HP1). HP1 can bridge chromatin segments and form liquid droplets, but the biophysical principles underlying heterochromatin compartmentalization in the cell nucleus are elusive. Here, we assess mechanistically relevant features of pericentric heterochromatin compaction in mouse fibroblasts. We find that (1) HP1 has only a weak capacity to form liquid droplets in living cells; (2) the size, global accessibility, and compaction of heterochromatin foci are independent of HP1; (3) heterochromatin foci lack a separated liquid HP1 pool; and (4) heterochromatin compaction can toggle between two "digital" states depending on the presence of a strong transcriptional activator. These findings indicate that heterochromatin foci resemble collapsed polymer globules that are percolated with the same nucleoplasmic liquid as the surrounding euchromatin, which has implications for our understanding of chromatin compartmentalization and its functional consequences.
Keywords: Heterochromatin protein 1; chromatin accessibility; chromatin compartmentalization; epigenetic editing; intracellular viscosity; liquid- liquid phase separation; nuclear organization; optodroplets; polarization-dependent fluorescence correlation spectroscopy; polymer collapse.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
Graphical abstract
Figure 1
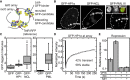
Droplet Formation of Recombinant Mouse HP1α in the Presence of DNA (A) Visualization of droplet formation by HP1α and GFP-HP1α when mixed with DNA. Arrows in the left and center panel highlight bona fide fusion intermediates. Scale bars, 5 μm. See also Figure S1. (B) Turbidity measurements for HP1α and GFP-HP1α in the presence of saturating amounts of DNA. Error bars represent SD from 3 replicates. The lines are Hill functions fitted to the data, assuming the same plateau value for both proteins. Fit parameters are listed in Table S1. (C) Visualization of droplet formation in mixtures of HP1α and GFP-HP1α (in the presence of DNA). The concentrations of GFP-HP1α amounted to 16 μM, 80 μM, 120 μM, and 144 μM (left to right). The total HP1 concentration in the samples was kept at ∼180 μM. Scale bars, 5 μm.
Figure 2
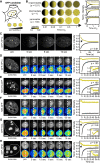
Internal Structure of Chromocenters (A) Distribution of HP1α in WT and Suv39h dn iMEF cells, visualized by immunostaining and STED nanoscopy. DNA was stained with DAPI and imaged by conventional confocal microscopy. The images in the first two rows have the same magnification. (B) Same as (A) but for H3K9me3. (C) Image correlation spectroscopy analysis of HP1α and H3K9me3 signals in chromocenters of iMEF WT and Suv39h dn cells. A total of 18 (H3K9me3, WT), 19 (H3K9me3, dn), 24 (HP1α, WT), and 14 (HP1α, dn) cells were analyzed. Error bars represent SEM. Solid lines represent fit functions (STAR Methods). (D) Quantitation of cluster size (left) and abundance (center) from fitting the correlation functions shown in (C) and pixel intensity distribution in chromocenters (right). Correlation functions for WT cells contained an additional large component and were fitted with double-exponential functions (STAR Methods; Table S2). Error bars represent standard fit errors. See also Figure S2 for a quantitative analysis of the compaction, number, and size of chromocenters in WT and Suv39h dn iMEF cells.
Figure 3
Formation and Stability of Optodroplets in Living Cells (A) Schematic representation of the optodroplet system and the experimental design. A protein of interest (“candidate”) is fused to PHR-mCherry, and its ability to form and stabilize droplets is evaluated by switching blue light on/off. (B) Localization and droplet formation of PHR-mCherry-HP1α in iMEF cells. Scale bar, 5 μm. (C) Same as (B) but for U2OS cells. (D) Droplet formation of PHR-mCherry-HP1α compared with PHR-mCherry in U2OS cells. Expression levels determined by FCS are indicated at the bottom right. Scale bars, 5 μm. (E) Concentration-dependent droplet formation capacity of PHR-mCherry alone (PHR-mCh) and of fusions of PHR-mCherry with HP1α, the dimerization-deficient mutant HP1α I163A, the PxVxL module of SENP7, FUSN-HP1α and nucleolin (NCL) in U2OS cells. Curves are shown as a guide to the eye. See also Figures S3A and S3B. (F) Stability of droplets containing fusions of PHR-mCherry with the indicated proteins in U2OS cells. Images represent the first frame after blue light exposure (+ blue light) and the time point 8 min after the light pulse (− blue light). NCL and NPM accumulate in nucleoli independently of blue light illumination, which leads to a strong signal in these regions. Error bars represent SEM of at least 10 replicates. Errors for half-lives represent standard fit errors. For RGG2-HP1 and FUSN-HP1, a subset of droplets persisted, as reflected by the plateaus (dashed lines). Scale bars, 5 μm. See also Figure S3C.
Figure 4
Capacity of HP1α and Other Candidates to Form Droplets When Tethered to Chromatin (A) Schematic representation of the _lac_O/_tet_O tethering system to test droplet formation. (B) Confocal microscopy images showing _lac_O arrays bound by the indicated proteins. The inset shows the _tet_O array bound by TetR and the _lac_O array bound by GFP-PML III. Scale bars, 5 μm. (C) Quantitation of GFP-tagged molecules at _lac_O arrays. Note the different scale for PML III. At least 15 cells were analyzed for each condition. (D) FRAP analysis of GFP-HP1α at the _lac_O array. From a fit to the data (gray line), a stably (58%) and a transiently (42%) bound fraction of HP1α were resolved. The transient fraction likely represents molecules that accumulate at the array via HP1-HP1 interactions. See also Figures S4A and S4B. (E) qPCR analysis of the transcriptional activity of the reporter located adjacent to the _lac_O/_tet_O array in cells expressing TetR-PHR-YFP-HP1α and BFP-LacI (mock) or BFP-LacI-VP16 (VP16). For the latter case, cells were treated either only with doxycycline (dox) or with dox and light to assess the repressive potential of HP1α tethering alone compared with HP1α optodroplet formation at the array. Error bars represent SEM from 3 replicates. See also Figure S4C.
Figure 5
Internal Mixing of Chromocenters and Nucleoli (A) Schematic representation of the apparent permeability p of subcompartments, which is a measure of the prevalence of internal mixing of proteins within the subcompartment in relation to exchange with the surrounding nucleoplasm. (B) Predicted temporal intensity evolution after having bleached one half of a circle surrounded by a boundary with permeability p. The time axis is divided by the diffusion time τD, making the plotted curves independent of the diffusion coefficient. At t/τD = 0.5 (highlighted time point), the intensity of the non-bleached half is visibly decreased if a boundary is present. (C) Half-nucleus bleach for cells expressing GFP-HP1α (n = 5). The arrow points to the intensity decrease in the non-bleached half that reflects preferential internal mixing. (D) Half-chromocenter bleach for cells expressing GFP-HP1α (n = 35). The inset shows the intensity of the non-bleached half during the first 20 s of the experiment. Magnified images were smoothed for clarity. See also Figure S5. (E) Same as (D) but for MECP2-GFP (n = 22). (F) Same as (D) but for H2B-GFP (n = 19). (G) Half-nucleolus bleach for cells expressing GFP-NCL (n = 28). The inset shows the intensity of the non-bleached half during the first 20 s of the experiment. The arrow points to the intensity decrease in the non-bleached half that reflects preferential internal mixing. (H) Same as (G) but for GFP-NPM (n = 14). Scale bars in (C)–(H), 5 μm.
Figure 6
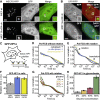
Accessibility and Local Viscosity of Chromocenters (A) Representative confocal images of WT and Suv39h dn cells expressing GFP and MECP2-RFP. Merge images: red, MECP2-RFP; green, GFP. Insets show magnified chromocenters with partial GFP exclusion. Scale bars, 5 μm. (B) Same as (A) but for Suv39h dn cells expressing RFP and MBD1-GFP (top) and for fixed and DAPI-stained Suv39h dn cells expressing GFP (bottom). (C) Schematic representation of the polarization-sensitive fluorescence correlation spectroscopy (Pol-FCS) experiment. Pol-FCS measures the local viscosity of chromocenters via rotational diffusion of GFP-HP1. HP1-HP1 interactions within a dense liquid phase formed by LLPS are expected to increase local viscosity. (D) Pol-FCS measurement of GFP-HP1 in living cells with crossed detectors to resolve only translational diffusion (n = 19). (E) Pol-FCS measurement of GFP-HP1 in living cells with parallel detectors to resolve both translational and rotational diffusion (n = 19; data for the detector configurations in this and in D were acquired in the same measurements). (F) Rotational diffusion times obtained from a fit to the Pol-FCS data shown in (E). Error bars represent standard fit errors. See also Table S4. (G) Pol-FCS measurement with parallel detectors of GFP-HP1 in glycerol/water mixtures with the indicated glycerol concentrations. (H) Rotational diffusion times obtained from fitting the Pol-FCS measurements in (G). Error bars represent standard fit errors. See also Figure S6 and Table S5.
Figure 7
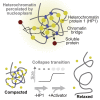
Regulation of Chromocenter Size and Compaction (A) Schematic representation of the plasmid used for HP1α overexpression and model predictions. Concentration buffering: HP1α overexpression increases chromocenter sizes, whereas HP1α levels inside and outside of chromocenters remain constant. Size buffering: chromocenters retain their size, whereas HP1α levels increase inside and outside of chromocenters. (B) Representative confocal images of cells expressing low (top) or high (bottom) levels of ZsGreen and HP1α. Scale bars, 5 μm. (C) HP1α levels inside and outside of chromocenters, DAPI levels at chromocenters, and chromocenter area as a function of HP1α overexpression. The groups with no, low, medium, and high ZsGreen levels contained 1406, 45, 24, and 13 cells, respectively. See also Figure S7A. (D) Schematic representation of the epigenetic editing experiment to study heterochromatin decondensation. A switch-like dose response with digital compaction states is indicative of a collapse transition. (E) Representative confocal images of cells with dCas9-GFP-VPR or dCas9-GFP (mock) bound to major satellite repeats (mSats). The white arrows highlight decondensed chromocenter structures. Scale bars, 5 μm. See also Figure S7D. (F) DAPI levels at chromocenters and chromocenter area as a function of dCas9 levels at major satellites in WT iMEF cells. The dCas9-mock group contained 155 cells; the groups with low, medium, and high dCas9-VPR levels contained 83, 42, and 83 cells, respectively. See also Figures S7B and S7C. (G) Relationship between dCas9-VPR recruitment and chromocenter decondensation in WT iMEF (red) and Suv39h dn iMEF (gray) cells. The dashed line represents a fit with an exponential functional (as a guide to the eye). See also Figure S7F. (H) Representative confocal images of dCas9-VPR-expressing cells with major satellites enriched for RNA polymerase II phosphorylated at serine 5 (Pol II S5P). Scale bar, 5 μm. See also Figure S7E. (I) Same as (F) but for Suv39h dn iMEF cells. The dCas9-mock group contained 148 cells; the groups with low, medium, and high dCas9-VPR levels contained 95, 48, and 95 cells, respectively. See also Figures S7B and S7C. (J) Proposed model for heterochromatin separation that recapitulates our data. The left and the right state correspond to the endogenous eu- and heterochromatin states, respectively.
Comment in
- Just Took a DNA Test, Turns Out 100% Not That Phase.
Gitler AD, Shorter J, Ha T, Myong S. Gitler AD, et al. Mol Cell. 2020 Apr 16;78(2):193-194. doi: 10.1016/j.molcel.2020.03.029. Mol Cell. 2020. PMID: 32302539
Similar articles
- HP1 reshapes nucleosome core to promote phase separation of heterochromatin.
Sanulli S, Trnka MJ, Dharmarajan V, Tibble RW, Pascal BD, Burlingame AL, Griffin PR, Gross JD, Narlikar GJ. Sanulli S, et al. Nature. 2019 Nov;575(7782):390-394. doi: 10.1038/s41586-019-1669-2. Epub 2019 Oct 16. Nature. 2019. PMID: 31618757 Free PMC article. - The Drosophila HP1 family is associated with active gene expression across chromatin contexts.
Schoelz JM, Feng JX, Riddle NC. Schoelz JM, et al. Genetics. 2021 Aug 26;219(1):iyab108. doi: 10.1093/genetics/iyab108. Genetics. 2021. PMID: 34849911 Free PMC article. - HP1-driven phase separation recapitulates the thermodynamics and kinetics of heterochromatin condensate formation.
Tortora MMC, Brennan LD, Karpen G, Jost D. Tortora MMC, et al. Proc Natl Acad Sci U S A. 2023 Aug 15;120(33):e2211855120. doi: 10.1073/pnas.2211855120. Epub 2023 Aug 7. Proc Natl Acad Sci U S A. 2023. PMID: 37549295 Free PMC article. - Heterochromatin organization and phase separation.
Zhang H, Qin W, Romero H, Leonhardt H, Cardoso MC. Zhang H, et al. Nucleus. 2023 Dec;14(1):2159142. doi: 10.1080/19491034.2022.2159142. Nucleus. 2023. PMID: 36710442 Free PMC article. Review.
Cited by
- Two-Parameter Mobility Assessments Discriminate Diverse Regulatory Factor Behaviors in Chromatin.
Lerner J, Gomez-Garcia PA, McCarthy RL, Liu Z, Lakadamyali M, Zaret KS. Lerner J, et al. Mol Cell. 2020 Aug 20;79(4):677-688.e6. doi: 10.1016/j.molcel.2020.05.036. Epub 2020 Jun 22. Mol Cell. 2020. PMID: 32574554 Free PMC article. - A simulation model of heterochromatin formation at submolecular detail.
Williams MR, Xiaokang Y, Hathaway NA, Kireev D. Williams MR, et al. iScience. 2022 Jun 14;25(7):104590. doi: 10.1016/j.isci.2022.104590. eCollection 2022 Jul 15. iScience. 2022. PMID: 35800764 Free PMC article. - Encounters in Three Dimensions: How Nuclear Topology Shapes Genome Integrity.
Sebastian R, Aladjem MI, Oberdoerffer P. Sebastian R, et al. Front Genet. 2021 Oct 21;12:746380. doi: 10.3389/fgene.2021.746380. eCollection 2021. Front Genet. 2021. PMID: 34745220 Free PMC article. Review. - A cyclin-dependent kinase-mediated phosphorylation switch of disordered protein condensation.
Valverde JM, Dubra G, Phillips M, Haider A, Elena-Real C, Fournet A, Alghoul E, Chahar D, Andrés-Sanchez N, Paloni M, Bernadó P, van Mierlo G, Vermeulen M, van den Toorn H, Heck AJR, Constantinou A, Barducci A, Ghosh K, Sibille N, Knipscheer P, Krasinska L, Fisher D, Altelaar M. Valverde JM, et al. Nat Commun. 2023 Oct 9;14(1):6316. doi: 10.1038/s41467-023-42049-0. Nat Commun. 2023. PMID: 37813838 Free PMC article. - Spatial Organization of Chromatin: Emergence of Chromatin Structure During Development.
Ghosh RP, Meyer BJ. Ghosh RP, et al. Annu Rev Cell Dev Biol. 2021 Oct 6;37:199-232. doi: 10.1146/annurev-cellbio-032321-035734. Epub 2021 Jul 6. Annu Rev Cell Dev Biol. 2021. PMID: 34228506 Free PMC article. Review.
References
- Bannister A.J., Zegerman P., Partridge J.F., Miska E.A., Thomas J.O., Allshire R.C., Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials