Clinical features and survival of patients with indolent systemic mastocytosis defined by the updated WHO classification - PubMed (original) (raw)
. 2020 Aug;75(8):1927-1938.
doi: 10.1111/all.14248. Epub 2020 Mar 16.
Wolfgang R Sperr 2, Lucie Nekvindová 3, Hanneke O Elberink 4, Karoline V Gleixner 2, Aleksandra Gorska 5, Magdalena Lange 6, Karin Hartmann 7 8, Anja Illerhaus 8, Massimiliano Bonifacio 9, Cecelia Perkins 10, Chiara Elena 11, Luca Malcovati 11, Anna B Fortina 12, Khalid Shoumariyeh 13, Mohamad Jawhar 14, Roberta Zanotti 9, Patrizia Bonadonna 15, Francesca Caroppo 12, Alexander Zink 16, Massimo Triggiani 17, Roberta Parente 17, Nikolas von Bubnoff 13, Akif S Yavuz 18, Hans Hägglund 19, Mattias Mattsson 19, Jens Panse 20, Nadja Jäkel 21, Alex Kilbertus 22, Olivier Hermine 23, Michel Arock 24, David Fuchs 25, Vito Sabato 26, Knut Brockow 16, Agnes Bretterklieber 27, Marek Niedoszytko 5, Björn van Anrooij 4, Andreas Reiter 14, Jason Gotlib 10, Hanneke C Kluin-Nelemans 28, Jiri Mayer 1, Michael Doubek 1, Peter Valent 2
Affiliations
- PMID: 32108361
- PMCID: PMC7115854
- DOI: 10.1111/all.14248
Clinical features and survival of patients with indolent systemic mastocytosis defined by the updated WHO classification
Jakub Trizuljak et al. Allergy. 2020 Aug.
Abstract
Background: In indolent systemic mastocytosis (ISM), several risk factors of disease progression have been identified. Previous studies, performed with limited patient numbers, have also shown that the clinical course in ISM is stable and comparable to that of cutaneous mastocytosis (CM). The aim of this project was to compare the prognosis of patients with ISM with that of patients with CM.
Methods: We employed a dataset of 1993 patients from the registry of the European Competence Network on Mastocytosis (ECNM) to compare outcomes of ISM and CM.
Results: We found that overall survival (OS) is worse in ISM compared to CM. Moreover, in patients with typical ISM, bone marrow mastocytosis (BMM), and smoldering SM (SSM), 4.1% of disease progressions have been observed (4.9% of progressions in typical ISM group, 1.7% in BMM, and 9.4% in SSM). Progressions to advanced SM were observed in 2.9% of these patients. In contrast, six patients with CM (1.7%) converted to ISM and no definitive progression to advanced SM was found. No significant differences in OS and event-free survival (EFS) were found when comparing ISM, BMM, and SSM. Higher risk of both progression and death was significantly associated with male gender, worse performance status, and organomegaly.
Conclusion: Our data confirm the clinical impact of the WHO classification that separates ISM from CM and from other SM variants.
Keywords: WHO classification; cutaneous mastocytosis; indolent systemic mastocytosis; prognostication; survival.
© 2020 EAACI and John Wiley and Sons A/S. Published by John Wiley and Sons Ltd.
Conflict of interest statement
Conflict of interest
WRS received honoraria from Novartis, Pfizer, AbbVie, Daiichi Sankyo, Amgen, Thermo Fisher, Diciphera, Incyte, Celgene, Jazz and travel grants from Pfizer and Roche. HOE received honoraria from ALK-Abelló, Chiesi, MEDA Pharma, Novartis, Blueprint. KVG received honoraria from Novartis, Roche, BMS, Sanofi, Incyte and travel grants from Roche and AbbVie. ML received honoraria from Novartis. KH received honoraria from Novartis, ALK, Blueprint, Deciphera and research funding from Euroimmun. MB received honoraria from Amgen, Incyte, Pfizer and research funding from Novartis. CE received honoraria from Novartis and Pfizer. MJ received honoraria from Novartis, Blueprint, Deciphera. RZ is consultant Deciphera and Novartis. AZ received honoraria or participated in trials from AbbVie, Almirall, Beiersdorf Dermo Medical, Bencard Allergie, BMS, Celgene, Eli Lilly, GSK, Janssen-Cilag, Miltenyi Biotec, Novartis, Sanofi-Aventis, Takeda Pharma. MT received honoraria from Deciphera, Blueprint, Novartis. NB received honoraria from Astra Zeneca, Amgen, Novartis and BMS and research funding from Novartis. JP received honoraria from Alexion, BMS, Boehringer Ingelheim, Grünenthal, MSD, Novartis, Pfizer, Chugai. DF received honoraria from Novartis, Pfizer, Roche travel grants from Roche. VS received honoraria from Novartis, Termofisher, Shire, Stallergens. KB received honoraria from Novartis, Phadia (Thermo Fisher), Meda, BioMarin Pharmaceutical Inc outside. BA received honoraria from Novartis. AR received honoraria from Novartis, BMS, Deciphera, Blueprint, Baxalta/Shire and research funding from Novartis. JG received honoraria from Blueprint, Deciphera, Gilead, Incyte, Novartis and research funding from Blueprint, Celgene, CTI BioPharma, Deciphera, Gilead, Incyte, Pharmacyclics, Promedior, Seattle Genetics. JM received honoraria from for Novartis, Gilead, BMS. MD received honoraria from for Roche, AbbVie, Novartis, Gilead, AOP Pharmaceuticals, Janssen-Cilag. PV has received honoraria from Novartis, Pfizer, Deciphera, Incyte, Blueprint, Celgene and research funds from Pfizer, Incyte, Celgene. JT, LN, AG, AI, CP, LM, ABF, FC, KS, RP, MN, PB, ASY, HH, MM, NJ, AK, OH, MA, AB, HCKN have nothing to disclose.
Figures
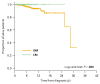
Figure 1
Overall survival (OS) of indolent systemic mastocytosis patients (ISM, n = 655) and cutaneous mastocytosis patients (CM, n = 242). P = .024 as determined by log-rank-test. Hazard ratio (HR) 0.0 (95% confidence interval: 0.0)
Similar articles
- Refined diagnostic criteria for bone marrow mastocytosis: a proposal of the European competence network on mastocytosis.
Zanotti R, Bonifacio M, Lucchini G, Sperr WR, Scaffidi L, van Anrooij B, Oude Elberink HN, Rossignol J, Hermine O, Gorska A, Lange M, Hadzijusufovic E, Miething C, Müller S, Perkins C, Shomali W, Elena C, Illerhaus A, Jawhar M, Parente R, Caroppo F, Solomianyi O, Zink A, Mattsson M, Yavuz AS, Panse J, Varkonyi J, Doubek M, Sabato V, Breynaert C, Vucinic V, Schug T, Hägglund H, Wortmann F, Brockow K, Angelova-Fischer I, Belloni Fortina A, Triggiani M, Reiter A, Hartmann K, Malcovati L, Gotlib J, Shoumariyeh K, Niedoszytko M, Arock M, Kluin-Nelemans HC, Bonadonna P, Valent P. Zanotti R, et al. Leukemia. 2022 Feb;36(2):516-524. doi: 10.1038/s41375-021-01406-y. Epub 2021 Sep 20. Leukemia. 2022. PMID: 34545185 - Prognostic Impact of Organomegaly in Mastocytosis: An Analysis of the European Competence Network on Mastocytosis.
Lübke J, Schwaab J, Christen D, Elberink HO, Span B, Niedoszytko M, Gorska A, Lange M, Gleixner KV, Hadzijusufovic E, Solomianyi O, Angelova-Fischer I, Zanotti R, Bonifacio M, Bonadonna P, Shoumariyeh K, von Bubnoff N, Müller S, Perkins C, Elena C, Malcovati L, Hagglund H, Mattsson M, Parente R, Varkonyi J, Fortina AB, Caroppo F, Zink A, Brockow K, Breynaert C, Bullens D, Yavuz AS, Doubek M, Sabato V, Schug T, Niederwieser D, Hartmann K, Triggiani M, Gotlib J, Hermine O, Arock M, Kluin-Nelemans HC, Panse J, Sperr WR, Valent P, Reiter A, Jawhar M. Lübke J, et al. J Allergy Clin Immunol Pract. 2023 Feb;11(2):581-590.e5. doi: 10.1016/j.jaip.2022.10.051. Epub 2022 Nov 17. J Allergy Clin Immunol Pract. 2023. PMID: 36403897 - Frequency and prognostic impact of blood-circulating tumor mast cells in mastocytosis.
Henriques A, Muñoz-González JI, Sánchez-Muñoz L, Matito A, Torres-Rivera L, Jara-Acevedo M, Caldas C, Mayado A, Pérez-Pons A, García-Montero AC, Álvarez-Twose I, Orfao A. Henriques A, et al. Blood. 2022 Jan 27;139(4):572-583. doi: 10.1182/blood.2021012694. Blood. 2022. PMID: 34496018 - Mastocytosis: state of the art.
Horny HP, Sotlar K, Valent P. Horny HP, et al. Pathobiology. 2007;74(2):121-32. doi: 10.1159/000101711. Pathobiology. 2007. PMID: 17587883 Review. - Diagnosis and management of mastocytosis: an emerging challenge in applied hematology.
Valent P. Valent P. Hematology Am Soc Hematol Educ Program. 2015;2015:98-105. doi: 10.1182/asheducation-2015.1.98. Hematology Am Soc Hematol Educ Program. 2015. PMID: 26637707 Review.
Cited by
- MASTering systemic mastocytosis: Lessons learned from a large patient cohort.
Tse KY, Chen W, Puttock EJ, Chowdhury S, Miller K, Powell D, Lampson B, Yuen C, Cattie D, Green T, Sullivan E, Zeiger RS. Tse KY, et al. J Allergy Clin Immunol Glob. 2024 Jul 27;3(4):100316. doi: 10.1016/j.jacig.2024.100316. eCollection 2024 Nov. J Allergy Clin Immunol Glob. 2024. PMID: 39234417 Free PMC article. - Standardized indolent systemic mastocytosis evaluations across a health care system: implications for screening accuracy.
McMurray JC, Pacheco CS, Schornack BJ, Sun X, Brunader JA, Scott AE, Ariza JS, Kou CJ, Costantino RC, Pittman LM, Adams KE, Waters AM, Pryor EM, Lyons JJ, Metcalfe DD, Maric I, Boggs NA. McMurray JC, et al. Blood. 2024 Jul 25;144(4):408-419. doi: 10.1182/blood.2023023347. Blood. 2024. PMID: 38635793 - [Mastocytosis-a frequently unrecognized disease].
Muñoz M, Siebenhaar F. Muñoz M, et al. Dermatologie (Heidelb). 2024 Jan;75(1):75-86. doi: 10.1007/s00105-023-05258-8. Epub 2023 Dec 12. Dermatologie (Heidelb). 2024. PMID: 38085334 German. - Review and Updates on Systemic Mastocytosis and Related Entities.
Li JY, Ryder CB, Zhang H, Cockey SG, Hyjek E, Moscinski LC, Sagatys E, Song J. Li JY, et al. Cancers (Basel). 2023 Nov 28;15(23):5626. doi: 10.3390/cancers15235626. Cancers (Basel). 2023. PMID: 38067330 Free PMC article. Review. - Secretory and Membrane-Associated Biomarkers of Mast Cell Activation and Proliferation.
Parente R, Giudice V, Cardamone C, Serio B, Selleri C, Triggiani M. Parente R, et al. Int J Mol Sci. 2023 Apr 11;24(8):7071. doi: 10.3390/ijms24087071. Int J Mol Sci. 2023. PMID: 37108232 Free PMC article. Review.
References
- Horny H-P, Akin C, Arber DA, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, LeBeau MM, Orazi A, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2017. pp. 61–70.
- Valent P, Horny H-P, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–605. - PubMed
- Sperr WR, Valent P. Diagnosis, progression patterns and prognostication in mastocytosis. Expert Rev Hematol. 2012;5:261–274. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources