The Epipeptide YydF Intrinsically Triggers the Cell Envelope Stress Response of Bacillus subtilis and Causes Severe Membrane Perturbations - PubMed (original) (raw)
The Epipeptide YydF Intrinsically Triggers the Cell Envelope Stress Response of Bacillus subtilis and Causes Severe Membrane Perturbations
Philipp F Popp et al. Front Microbiol. 2020.
Abstract
The Gram-positive model organism and soil bacterium Bacillus subtilis naturally produces a variety of antimicrobial peptides (AMPs), including the ribosomally synthesized and post-translationally modified AMP YydF, which is encoded in the yydFGHIJ locus. The yydF gene encodes the pre-pro-peptide, which is, in a unique manner, initially modified at two amino acid positions by the radical SAM epimerase YydG. Subsequently, the membrane-anchored putative protease YydH is thought to cleave and release the mature AMP, YydF, to the environment. The AMP YydF, with two discreet epimerizations among 17 residues as sole post-translational modification, defines a novel class of ribosomally synthesized and post-translationally modified peptides (RiPPs) called epipeptides, for which the mode-of-action (MOA) is unknown. The predicted ABC transporter encoded by yydIJ was previously postulated as an autoimmunity determinant of B. subtilis against its own AMP. Here, we demonstrate that extrinsically added YydF* kills B. subtilis cells by dissipating membrane potential via membrane permeabilization. This severe membrane perturbation is accompanied by a rapid reduction of membrane fluidity, substantiated by lipid domain formation. The epipeptide triggers a narrow and highly specific cellular response. The strong induction of liaIH expression, a marker for cell envelope stress in B. subtilis, further supports the MOA described above. A subsequent mutational study demonstrates that LiaIH-and not YydIJ-represents the most efficient resistance determinant against YydF* action. Unexpectedly, none of the observed cellular effects upon YydF* treatment alone are able to trigger liaIH expression, indicating that only the unique combination of membrane permeabilization and membrane rigidification caused by the epipetide, leads to the observed cell envelope stress response.
Keywords: Bacillus subtilis; RiPP; antimicrobial peptides; cell envelope stress response; epipeptide; membrane depolarization; membrane rigidity.
Copyright © 2020 Popp, Benjdia, Strahl, Berteau and Mascher.
Figures
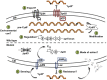
Figure 1
Schematic overview of the yydFGHIJ operon in B. subtilis. In the wild type (1–4), at transition from exponential to stationary phase a yet unknown cue triggers expression of the yyd operon (1). P_yydF_ is described as a constitutive σA-dependent promoter, details on any regulation beyond that are still missing (2) (Butcher et al., 2007). The radical SAM epimerase YydG post-translationally modifies pre-YydF by substituting two amino acids from their L-form into their D-counterparts (3) (Benjdia et al., 2017b). Export and further processing of pre-YydF to its active from is most likely mediated by the membrane bound protease YydH (4). The ABC transporter YydIJ is postulated as potential resistance determinant (red). In a yydIJ knockout strain (5–7), active YydF* launches the LiaRS TCS (5) (Butcher et al., 2007). The phage-shock like protein LiaH and its anchor protein LiaI, are potentially involved in mediating resistance against YydF* (6). Detailed investigations if and how YydF* kills B. subtilis cells are yet to be unraveled (7). Arrows indicate activation, T-bars inhibition; CM, cytoplasmic membrane.
Figure 2
Growth curves and luciferase activity of B. subtilis P_liaI_-lux strains. Upper panels depict growth curves, lower panels show luminescence values normalized over optical density. (A) Growth in minimal media of P_liaI_-lux strains in the wild type background and individual yyd mutants. (B) Wild type P_liaI_-lux (red) and an empty vector control (blue) incubated in full medium, were challenged with extrinsic added 0.5 μM YydF* (full lines) or non-treated (dotted lines).
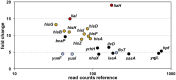
Figure 3
RNA-sequencing (RNA-seq) profile of B. subtilis upon YydF* treatment. Visualization of altered gene expression in B. subtilis upon 0.5 μM YydF* exposure after 10 min, compared to non-induced control samples. Fold change of elevated RNA-seq counts are plotted over read counts in the reference condition. Same colored dots highlight genes encoded within an operon. For further details, see Table 1 and Table S2.
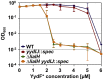
Figure 4
Minimum inhibitory concentration (MIC) assay of B. subtilis treated with YydF*. Optical density endpoint measurements after 24 h are plotted over the indicated YydF* concentrations. Next to the wild type (blue), a yydIJ mutant (red), a liaIH knockout strain (green), and the corresponding double mutant (orange) are depicted.
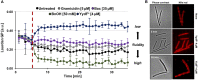
Figure 5
YydF* causes membrane depolarization and membrane permeabilization. (A) Membrane potential levels of B. subtilis cells upon treatment with different antimicrobial compounds were obtained using the voltage sensitive dye DiSC3(5). Gramicidin (5 μM, ~10xMIC) and nisin (~MIC) (gray and gold lines) were used as positive controls for rapid and complete membrane depolarization. Addition of 4 μM YydF* showed a rapid depolarization in the first 5 min after addition, followed by a gradual complete depolarization. The peptide antibiotic bacitracin at 35 μM and non-treated cells showed no membrane depolarization (purple and black lines). (B) Membrane potential and permeability measurements on single-cell level. Phase contrast (left panels) and fluorescence microscopy of B. subtilis cells stained with DiSC3(5) (middle panels) as well as with the membrane permeability indicator sytox Green (right panels). (C) Quantification of DiSC3(5) and sytox green fluorescence from individual cells per tested condition (~300), derived from the fluorescence microscopy experiments in (B). Membrane depolarization yields in low DiSC3(5) fluorescence, whereas membrane permeabilization results in high sytox green signal.
Figure 6
YydF* causes membrane rigidification and lipid domain formation. (A) Measurement of generalized polarization (GP) values using the fluidity sensitive fluorescent probe laurdan. Higher laurdan GP values correlate with rigid membrane state. Cells were incubated for 5 min to obtain basal GP levels, followed by addition of compounds (dashed red line). Benzyl alcohol was used as positive control for increased membrane fluidity, i.e., drop of GP values (green line). Non-treated, gramicidin, and bacitracin showed no effects on the fluidity state of the membrane (black, gray, and purple lines). Addition of 4 μM YydF* lead to a rigidification of B. subtilis cell membranes, indicated by elevated GP values (blue line). (B) Phase contrast and fluorescence images of B. subtilis cells stained with the membrane dye nile red after 5 and 20 min incubation with 4 μM YydF*, respectively.
Similar articles
- The Epipeptide Biosynthesis Locus epeXEPAB Is Widely Distributed in Firmicutes and Triggers Intrinsic Cell Envelope Stress.
Popp PF, Friebel L, Benjdia A, Guillot A, Berteau O, Mascher T. Popp PF, et al. Microb Physiol. 2021;31(3):306-318. doi: 10.1159/000516750. Epub 2021 Jun 11. Microb Physiol. 2021. PMID: 34120110 - The yydFGHIJ operon of Bacillus subtilis encodes a peptide that induces the LiaRS two-component system.
Butcher BG, Lin YP, Helmann JD. Butcher BG, et al. J Bacteriol. 2007 Dec;189(23):8616-25. doi: 10.1128/JB.01181-07. Epub 2007 Oct 5. J Bacteriol. 2007. PMID: 17921301 Free PMC article. - Post-translational modification of ribosomally synthesized peptides by a radical SAM epimerase in Bacillus subtilis.
Benjdia A, Guillot A, Ruffié P, Leprince J, Berteau O. Benjdia A, et al. Nat Chem. 2017 Jul;9(7):698-707. doi: 10.1038/nchem.2714. Epub 2017 Feb 6. Nat Chem. 2017. PMID: 28644475 Free PMC article. - Antimicrobial Activity of Cationic Antimicrobial Peptides against Gram-Positives: Current Progress Made in Understanding the Mode of Action and the Response of Bacteria.
Omardien S, Brul S, Zaat SA. Omardien S, et al. Front Cell Dev Biol. 2016 Oct 14;4:111. doi: 10.3389/fcell.2016.00111. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27790614 Free PMC article. Review. - Genetics of subtilin and nisin biosyntheses: biosynthesis of lantibiotics.
Entian KD, de Vos WM. Entian KD, et al. Antonie Van Leeuwenhoek. 1996 Feb;69(2):109-17. doi: 10.1007/BF00399416. Antonie Van Leeuwenhoek. 1996. PMID: 8775971 Review.
Cited by
- Structural and mechanistic basis for RiPP epimerization by a radical SAM enzyme.
Kubiak X, Polsinelli I, Chavas LMG, Fyfe CD, Guillot A, Fradale L, Brewee C, Grimaldi S, Gerbaud G, Thureau A, Legrand P, Berteau O, Benjdia A. Kubiak X, et al. Nat Chem Biol. 2024 Mar;20(3):382-391. doi: 10.1038/s41589-023-01493-1. Epub 2023 Dec 29. Nat Chem Biol. 2024. PMID: 38158457 - Radical SAM Enzymes and Ribosomally-Synthesized and Post-translationally Modified Peptides: A Growing Importance in the Microbiomes.
Benjdia A, Berteau O. Benjdia A, et al. Front Chem. 2021 Jul 19;9:678068. doi: 10.3389/fchem.2021.678068. eCollection 2021. Front Chem. 2021. PMID: 34350157 Free PMC article. Review. - Metabolome Analysis of Constituents in Membrane Vesicles for Clostridium thermocellum Growth Stimulation.
Ichikawa S, Tsuge Y, Karita S. Ichikawa S, et al. Microorganisms. 2021 Mar 13;9(3):593. doi: 10.3390/microorganisms9030593. Microorganisms. 2021. PMID: 33805707 Free PMC article. - Biosynthesis of the sactipeptide Ruminococcin C by the human microbiome: Mechanistic insights into thioether bond formation by radical SAM enzymes.
Balty C, Guillot A, Fradale L, Brewee C, Lefranc B, Herrero C, Sandström C, Leprince J, Berteau O, Benjdia A. Balty C, et al. J Biol Chem. 2020 Dec 4;295(49):16665-16677. doi: 10.1074/jbc.RA120.015371. Epub 2020 Sep 24. J Biol Chem. 2020. PMID: 32972973 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases