Inhibitory Effects of a Reengineered Anthrax Toxin on Canine Oral Mucosal Melanomas - PubMed (original) (raw)
doi: 10.3390/toxins12030157.
Marcia Kazumi Nagamine 1, Ivone Izabel Mackowiak da Fonseca 1, Andrea Caringi Miraldo 1, Nayra Villar Scattone 1, José Luiz Guerra 1, José Guilherme Xavier 2, Mário Santos 2, Cristina Oliveira Massoco de Salles Gomes 1, Jerrold Michael Ward 3, Shihui Liu 4, Stephen Howard Leppla 5, Thomas Henrik Bugge 6, Maria Lucia Zaidan Dagli 1
Affiliations
- PMID: 32121654
- PMCID: PMC7150776
- DOI: 10.3390/toxins12030157
Inhibitory Effects of a Reengineered Anthrax Toxin on Canine Oral Mucosal Melanomas
Adriana Tomoko Nishiya et al. Toxins (Basel). 2020.
Abstract
Canine oral mucosal melanomas (OMM) are the most common oral malignancy in dogs and few treatments are available. Thus, new treatment modalities are needed for this disease. Bacillus anthracis (anthrax) toxin has been reengineered to target tumor cells that express urokinase plasminogen activator (uPA) and metalloproteinases (MMP-2), and has shown antineoplastic effects both, in vitro and in vivo. This study aimed to evaluate the effects of a reengineered anthrax toxin on canine OMM. Five dogs bearing OMM without lung metastasis were included in the clinical study. Tumor tissue was analyzed by immunohistochemistry for expression of uPA, uPA receptor, MMP-2, MT1-MMP and TIMP-2. Animals received either three or six intratumoral injections of the reengineered anthrax toxin prior to surgical tumor excision. OMM samples from the five dogs were positive for all antibodies. After intratumoral treatment, all dogs showed stable disease according to the canine Response Evaluation Criteria in Solid Tumors (cRECIST), and tumors had decreased bleeding. Histopathology has shown necrosis of tumor cells and blood vessel walls after treatment. No significant systemic side effects were noted. In conclusion, the reengineered anthrax toxin exerted inhibitory effects when administered intratumorally, and systemic administration of this toxin is a promising therapy for canine OMM.
Keywords: Bacillus anthracis; anthrax; dog; oral melanoma; toxin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Figure 1
Tumoral volume of canine OMM in 5 dogs after intratumoral treatment with the reengineered anthrax toxin.
Figure 2
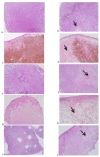
Photomicrographs of canine OMM biopsy samples from dogs 1 (a,b), 2 (c,d), 3 (e,f), 4 (g,h) and 5 (i,j), obtained before (a,c,e,g,i) and after (b,d,f,h,j) the intratumoral treatment with the reengineered anthrax toxin. In samples obtained after the treatment, it is possible to see necrotic areas (arrows), as well as edema and hemorrhage. All photomicrographs were obtained in a stereo microscope (Nikon®), with a magnification of 5×. (H&E, Scale bar = 0.5 mm.).
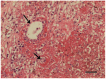
Figure 3
Detail of histopathological sample of OMM from dog 4, showing necrosis of the blood vessel walls (arrows). (H&E, Scale bar = 100 μm.).
Similar articles
- Inhibitory Effects of a Reengineered Anthrax Toxin on Canine and Human Osteosarcoma Cells.
Mackowiak da Fonseca J, Mackowiak da Fonseca II, Nagamine MK, Massoco CO, Nishiya AT, Ward JM, Liu S, Leppla SH, Bugge TH, Dagli MLZ. Mackowiak da Fonseca J, et al. Toxins (Basel). 2020 Sep 24;12(10):614. doi: 10.3390/toxins12100614. Toxins (Basel). 2020. PMID: 32987941 Free PMC article. - Comparative toxicity and efficacy of engineered anthrax lethal toxin variants with broad anti-tumor activities.
Peters DE, Hoover B, Cloud LG, Liu S, Molinolo AA, Leppla SH, Bugge TH. Peters DE, et al. Toxicol Appl Pharmacol. 2014 Sep 1;279(2):220-9. doi: 10.1016/j.taap.2014.06.010. Epub 2014 Jun 24. Toxicol Appl Pharmacol. 2014. PMID: 24971906 Free PMC article. - Potent antitumor activity of a urokinase-activated engineered anthrax toxin.
Liu S, Aaronson H, Mitola DJ, Leppla SH, Bugge TH. Liu S, et al. Proc Natl Acad Sci U S A. 2003 Jan 21;100(2):657-62. doi: 10.1073/pnas.0236849100. Epub 2003 Jan 13. Proc Natl Acad Sci U S A. 2003. PMID: 12525700 Free PMC article. - The Comparative Oncology of Canine Malignant Melanoma in Targeted Therapy: A Systematic Review of In Vitro Experiments and Animal Model Reports.
He X, Gao Y, Deng Y, He J, Nolte I, Murua Escobar H, Yu F. He X, et al. Int J Mol Sci. 2024 Sep 26;25(19):10387. doi: 10.3390/ijms251910387. Int J Mol Sci. 2024. PMID: 39408717 Free PMC article. Review. - Anthrax fusion protein therapy of cancer.
Frankel AE, Powell BL, Duesbery NS, Vande Woude GF, Leppla SH. Frankel AE, et al. Curr Protein Pept Sci. 2002 Aug;3(4):399-407. doi: 10.2174/1389203023380567. Curr Protein Pept Sci. 2002. PMID: 12370003 Review.
Cited by
- The Urokinase Receptor (uPAR) as a "Trojan Horse" in Targeted Cancer Therapy: Challenges and Opportunities.
Metrangolo V, Ploug M, Engelholm LH. Metrangolo V, et al. Cancers (Basel). 2021 Oct 27;13(21):5376. doi: 10.3390/cancers13215376. Cancers (Basel). 2021. PMID: 34771541 Free PMC article. Review. - An in vivo selection-derived d-peptide for engineering erythrocyte-binding antigens that promote immune tolerance.
Loftis AR, Zhang G, Backlund C, Quartararo AJ, Pishesha N, Hanna CC, Schissel CK, Garafola D, Loas A, Collier RJ, Ploegh H, Irvine DJ, Pentelute BL. Loftis AR, et al. Proc Natl Acad Sci U S A. 2021 Aug 24;118(34):e2101596118. doi: 10.1073/pnas.2101596118. Proc Natl Acad Sci U S A. 2021. PMID: 34417313 Free PMC article. - Targeting canine mammary neoplastic epithelial cells with a reengineered anthrax toxin: first study.
da Fonseca IIM, Nagamine MK, Gentile LB, Nishiya AT, da Fonseca JM, de Oliveira Massoco C, Ward JM, Liu S, Leppla SH, Dagli MLZ. da Fonseca IIM, et al. Vet Res Commun. 2024 Aug;48(4):2407-2428. doi: 10.1007/s11259-024-10400-5. Epub 2024 May 28. Vet Res Commun. 2024. PMID: 38805149 - RAGE Signaling in Melanoma Tumors.
Olaoba OT, Kadasah S, Vetter SW, Leclerc E. Olaoba OT, et al. Int J Mol Sci. 2020 Nov 26;21(23):8989. doi: 10.3390/ijms21238989. Int J Mol Sci. 2020. PMID: 33256110 Free PMC article. Review. - Inhibitory Effects of a Reengineered Anthrax Toxin on Canine and Human Osteosarcoma Cells.
Mackowiak da Fonseca J, Mackowiak da Fonseca II, Nagamine MK, Massoco CO, Nishiya AT, Ward JM, Liu S, Leppla SH, Bugge TH, Dagli MLZ. Mackowiak da Fonseca J, et al. Toxins (Basel). 2020 Sep 24;12(10):614. doi: 10.3390/toxins12100614. Toxins (Basel). 2020. PMID: 32987941 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous