Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression - PubMed (original) (raw)
Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression
Fang Liu et al. Int J Infect Dis. 2020 Jun.
Abstract
Objectives: To explore the epidemiological information, clinical characteristics, therapeutic outcomes and temporal progression of laboratory findings in 2019-coronavirus disease (COVID-19) patients exposed to lopinavir.
Methods: We collected data from ten COVID-19 patients admitted between January 22, 2020 and February 11, 2020 at Xixi hospital in Hangzhou, China.
Results: Of ten patients, secondary, tertiary and quartus patients emerged; the incubation period was 3-7 days. Mainly initial symptoms were cough and low fever (37.3-38.0°C). An asymptomatic case presented normal radiography, the others had ground glass opacities. All cases (three transferred, seven discharged) were exposed to lopinavir on initial hospitalization. Three patients stopped lopinavir because of adverse effects, two of them deteriorated, one was hospitalized longer than others who with sustained lopinavir use. Levels of potassium, albumin, and lymphocytes were low, but increased persistently after treatment. Eosinophil values were low on initial hospitalization, then all returned to normal before discharge. Viral load of SARS-CoV-2, radiography and eosinophil improved continuously in 3-14, 6-8 and 7-9 days, respectively.
Conclusions: Increasing eosinophils may be an indicator of COVID-19 improvement. The COVID-19 patients may benefit from sustained lopinavir use. More research on a larger scale is needed to verify these points.
Keywords: 2019-Coronavirus disease; Asymptomatic infection; Eosinophil; Lopinavir.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
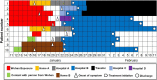
Figure 1
Contact history and timeline of the onset of symptoms in COVID-19 patients.
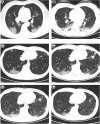
Figure 2
Chest radiographs showing patchy ground-glass opacities (panel A: patient 3), patchy hyperdense areas (panel B: patient 1) and multifocal opacity changes over time (panel C: patient 8).
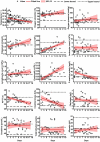
Figure 3
The temporal changes of laboratory indicators in COVID-19 patients after initiation of LPV-combined therapy.
Figure 4
The changes over time on eosinophil level and SARS-CoV-2 viral load in each COVID-19 patient.
Similar articles
- Safety and efficacy of antiviral combination therapy in symptomatic patients of Covid-19 infection - a randomised controlled trial (SEV-COVID Trial): A structured summary of a study protocol for a randomized controlled trial.
Panda PK, Bandyopadhyay A, Singh BC, Moirangthem B, Chikara G, Saha S, Bahurupi YA. Panda PK, et al. Trials. 2020 Oct 20;21(1):866. doi: 10.1186/s13063-020-04774-5. Trials. 2020. PMID: 33081849 Free PMC article. Clinical Trial. - An investigation into the beneficial effects of high-dose interferon beta 1-a, compared to low-dose interferon beta 1-a (the base therapeutic regimen) in moderate to severe COVID-19: A structured summary of a study protocol for a randomized controlled l trial.
Alavi Darazam I, Hatami F, Rabiei MM, Pourhoseingholi MA, Moradi O, Shokouhi S, Hajesmaeili MR, Shabani M, Irvani SSN. Alavi Darazam I, et al. Trials. 2020 Oct 26;21(1):880. doi: 10.1186/s13063-020-04812-2. Trials. 2020. PMID: 33106183 Free PMC article. Clinical Trial. - Lopinavir pharmacokinetics in COVID-19 patients.
Gregoire M, Le Turnier P, Gaborit BJ, Veyrac G, Lecomte R, Boutoille D, Canet E, Imbert BM, Bellouard R, Raffi F. Gregoire M, et al. J Antimicrob Chemother. 2020 Sep 1;75(9):2702-2704. doi: 10.1093/jac/dkaa195. J Antimicrob Chemother. 2020. PMID: 32443151 Free PMC article. No abstract available. - BET 1: Lopinavir-ritonavir and COVID-19.
Dolan D, Ingham J, Baombe J. Dolan D, et al. Emerg Med J. 2020 Jul;37(7):450-451. doi: 10.1136/emermed-2020-210221.2. Emerg Med J. 2020. PMID: 32616658 Review. No abstract available. - Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2.
Uzunova K, Filipova E, Pavlova V, Vekov T. Uzunova K, et al. Biomed Pharmacother. 2020 Nov;131:110668. doi: 10.1016/j.biopha.2020.110668. Epub 2020 Aug 24. Biomed Pharmacother. 2020. PMID: 32861965 Free PMC article. Review.
Cited by
- A prediction model for COVID-19 liver dysfunction in patients with normal hepatic biochemical parameters.
Bao J, Liu S, Liang X, Wang C, Cao L, Li Z, Wei F, Fu A, Shi Y, Shen B, Zhu X, Zhao Y, Liu H, Miao L, Wang Y, Liang S, Wu L, Huang J, Guo T, Liu F. Bao J, et al. Life Sci Alliance. 2022 Oct 19;6(1):e202201576. doi: 10.26508/lsa.202201576. Print 2023 Jan. Life Sci Alliance. 2022. PMID: 36261228 Free PMC article. - SARS-CoV-2 detection, viral load and infectivity over the course of an infection.
Walsh KA, Jordan K, Clyne B, Rohde D, Drummond L, Byrne P, Ahern S, Carty PG, O'Brien KK, O'Murchu E, O'Neill M, Smith SM, Ryan M, Harrington P. Walsh KA, et al. J Infect. 2020 Sep;81(3):357-371. doi: 10.1016/j.jinf.2020.06.067. Epub 2020 Jun 29. J Infect. 2020. PMID: 32615199 Free PMC article. - COVID-19: Rational discovery of the therapeutic potential of Melatonin as a SARS-CoV-2 main Protease Inhibitor.
Feitosa EL, Júnior FTDSS, Nery Neto JAO, Matos LFL, Moura MHS, Rosales TO, De Freitas GBL. Feitosa EL, et al. Int J Med Sci. 2020 Jul 30;17(14):2133-2146. doi: 10.7150/ijms.48053. eCollection 2020. Int J Med Sci. 2020. PMID: 32922174 Free PMC article. - Interferon therapy in patients with SARS, MERS, and COVID-19: A systematic review and meta-analysis of clinical studies.
Saleki K, Yaribash S, Banazadeh M, Hajihosseinlou E, Gouravani M, Saghazadeh A, Rezaei N. Saleki K, et al. Eur J Pharmacol. 2021 Sep 5;906:174248. doi: 10.1016/j.ejphar.2021.174248. Epub 2021 Jun 12. Eur J Pharmacol. 2021. PMID: 34126092 Free PMC article. - Interim Guidelines on Antiviral Therapy for COVID-19.
Kim SB, Huh K, Heo JY, Joo EJ, Kim YJ, Choi WS, Kim YJ, Seo YB, Yoon YK, Ku NS, Jeong SJ, Kim SH, Peck KR, Yeom JS. Kim SB, et al. Infect Chemother. 2020 Jun;52(2):281-304. doi: 10.3947/ic.2020.52.2.281. Epub 2020 Apr 23. Infect Chemother. 2020. PMID: 32342676 Free PMC article. Review.
References
- Dybul M., Fauci A.S., Bartlett J.G., Kaplan J.E., Pau A.K. Guidelines for Using Antiretroviral Agents among HIV-Infected Adults and Adolescents: The Panel on Clinical Practices for Treatment of HIV*. Ann Intern Med. 2002;51(RR-7):1–55. - PubMed
- Hamp D.B., Leženić R., Duper H.M., Goranović T. Respiratory failure as a result of chronic impaired ventilation in actualization of insufficient respiration: a case report. Trends Anaesth Crit Care. 2017;16:32–33.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous