Rg1 improves LPS-induced Parkinsonian symptoms in mice via inhibition of NF-κB signaling and modulation of M1/M2 polarization - PubMed (original) (raw)
Rg1 improves LPS-induced Parkinsonian symptoms in mice via inhibition of NF-κB signaling and modulation of M1/M2 polarization
Jia-Qi Liu et al. Acta Pharmacol Sin. 2020 Apr.
Abstract
Ginsenoside Rg1 is one of the most active ingredients in ginseng, which has been reported to protect dopaminergic neurons and improve behavioral defects in MPTP model, 6-OHDA model and rotenone model. However, it is unclear whether Rg1 exerted neuroprotection in LPS-induced sub-acute PD model. In this study, we investigated the neuroprotective effect of Rg1 in the sub-acute PD mouse model and explored the related mechanisms. Rg1 (10, 20, 40 mg·kg-1·d-1) was orally administered to mice for 18 days. A sub-acute PD model was established in the mice through LPS microinjection into the substantia nigra (SN) from D8 to D13. We found that Rg1 administration dose-dependently inhibited LPS-induced damage of dopaminergic neurons and activation of glial cells in the substantia nigra pars compacta (SNpc). The neuroprotective effects of Rg1 were associated with the reduction of pro-inflammatory cytokines and the improvement of anti-inflammatory cytokines and neurotrophin in the midbrain. Rg1 shifted the polarization of microglia towards the M2 phenotype from M1, evidenced by decreased M1 markers (inducible NO synthase, CD16, etc.) and increased M2 markers (arginase 1 (Arg1), CD206, etc) in the midbrain. Furthermore, Rg1 administration markedly inhibited nuclear translocation of NF-κB in midbrain microglia. In conclusion, Rg1 protects PD mice induced by continuous LPS injection by inhibiting the nuclear entry of NF-κB and regulating the polarization balance of microglia, shedding new light on a disease-modifying therapy of PD.
Keywords: NF-κB; Parkinson’s disease; ginsenoside Rg1; lipopolysaccharide; microglia polarization; neuroinflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures
Fig. 1
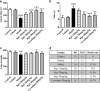
Scheme of the experiment procedure. After seven days of acclimatization, the mice were pretrained in three behavior tests. The mice were then randomly divided into seven experimental groups. One hour prior to the guide cannula implantation, the mice of Rg1 treatment groups and levodopa group were orally administered Rg1 or levodopa, respectively, from D0 to D18. The mice, except the control group, were implanted with two-sided hole guide cannulas. Eight days after surgery, the mice implanted with cannulas were injected bilaterally with LPS or saline, from D8 to D13. From D14 to D17, a series of behavior tests were conducted. On D18, all mice were sacrificed for further study
Fig. 2
Neuroprotective effect of Rg1 against the LPS-induced death rate and behavior defects. a Protective effect of Rg1 on motor coordination in the rotarod test. b Protective effect of Rg1 on bradykinesia in the pole test. c Protective effect of Rg1 in the grip strength test. d Animal death rate across experimental groups. ##P < 0.01, ###P < 0.001 vs. vehicle group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. model group. All data were presented as mean ± SD of triplicate independent experiments
Fig. 3
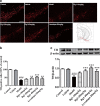
Rg1 restored the reduction of TH-positive neurons and protein expression in the substantia nigra pars compacta (SNpc) induced by LPS. a Representative immunofluorescence images of TH-immunoreactive neurons across groups (scale bar = 100 μm). b A histogram representing the quantitative analysis of TH-positive cells normalized to control levels is shown. Representative protein bands of TH and b-actin and histogram representing the quantitative analysis of TH levels normalized to β-actin protein levels are shown. c Representative protein bands of TH and β-actin and the histograms representing the quantitative analysis of TH levels normalized to β-actin protein levels are shown. All data are presented as mean ± SD of triplicate independent experiments. ###P < 0.001 vs. vehicle group; **P < 0.01, ***P < 0.001 vs. model group. All data are presented as mean ± SD of triplicate independent experiments
Fig. 4
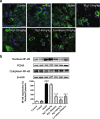
Rg1 attenuates glial activation induced by LPS in the SNpc. a, d Representative immunofluorescence images of IBA-1 and GFAP immunoreactive cells in the SNpc (scale bar = 20 μm). b, e Two histograms representing the respective quantitative analysis of IBA-1-positive and GFAP-positive cells normalized to control levels are shown. c, f Representative protein bands of IBA-1, GFAP, and β-actin and the histograms representing the quantitative analysis of IBA-1 or GFAP levels normalized to β-actin protein levels are shown. All data were presented as mean ± SD of triplicate independent experiments. ###P < 0.001 vs. vehicle group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. model group
Fig. 5
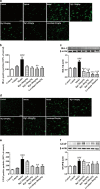
Rg1 alleviates the inflammatory response in LPS-injured PD mice. a Expression of TNF-α, IL-1β, IL-6, and IL-10 in the mice midbrain were determined using qPCR. b Representative blots and densitometry data for TNF-α, IL-1β, IL-6, IL-10, BDNF, and TGF-β. All data were presented as mean ± SD of triplicate independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. vehicle group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. model group
Fig. 6
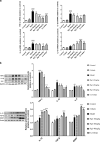
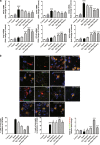
Rg1 regulated the M1 and M2 activation states. a qPCR analysis of mRNA expression of M1 markers (iNOS, CD16, CD32) and M2 markers (Arg1, CCL-22, TGF-β) in the midbrain of LPS-PD mice. b Representative photomicrographs of double-staining immunofuorescence of CD16/32 with Iba-1 and CD206 with Iba-1 in the midbrain of LPS-induced PD mice. Quantitative analysis of CD16/32-positive and CD206-positive microglia in the midbrain (scale bar = 10 μm). All data were presented as mean ± SD of triplicate independent experiments. #P < 0.05, ###P < 0.001 vs. vehicle group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. model group
Fig. 7
Effect of Rg1 on the transcriptional activity of NF-κB. a Translocation of NF-κB toward the nucleus determined by Immunofluorescence staining (scale bars = 10 μm). b Representative blots and densitometry data for nucleus/cytoplasm NF-κB ratio in the midbrain microglia. All data were presented as mean ± SD of triplicate independent experiments. ###P < 0.001 vs. vehicle group; ***P < 0.001 vs. model group
Fig. 8
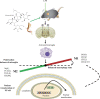
Schematic diagram of the neuroprotective effect of Rg1 in LPS-induced subacute PD mice by microglial polarization regulation. Continuous injection of LPS into the SN of mice resulted in polarization of activated microglia cells in the midbrain to pro-inflammatory M1 phenotype, accompanied by increased expression of markers (iNOS, CD16, CD32, and CD16/32). Rg1 modulates microglia toward a lower M1 phenotype polarization and higher M2 phenotype polarization in the SN of PD mice, which was accompanied by reducing the nuclear translocation of NF-κB, leading to increased expression of markers (Arg1, CCL22, TGF-β, and CD206) and improvement of PD-like symptoms
Similar articles
- Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting α-synuclein abnormalities in the substantia nigra.
Heng Y, Zhang QS, Mu Z, Hu JF, Yuan YH, Chen NH. Heng Y, et al. Toxicol Lett. 2016 Jan 22;243:7-21. doi: 10.1016/j.toxlet.2015.12.005. Epub 2015 Dec 23. Toxicol Lett. 2016. PMID: 26723869 - Farrerol protects dopaminergic neurons in a rat model of lipopolysaccharide-induced Parkinson's disease by suppressing the activation of the AKT and NF-κB signaling pathways.
Li Y, Zeng Y, Meng T, Gao X, Huang B, He D, Ran X, Du J, Zhang Y, Fu S, Hu G. Li Y, et al. Int Immunopharmacol. 2019 Oct;75:105739. doi: 10.1016/j.intimp.2019.105739. Epub 2019 Jul 24. Int Immunopharmacol. 2019. PMID: 31351366 - Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra.
Sun XC, Ren XF, Chen L, Gao XQ, Xie JX, Chen WF. Sun XC, et al. J Steroid Biochem Mol Biol. 2016 Jan;155(Pt A):94-103. doi: 10.1016/j.jsbmb.2015.09.040. Epub 2015 Oct 9. J Steroid Biochem Mol Biol. 2016. PMID: 26455404 - Nuclear Factor Kappa B: A Nobel Therapeutic Target of FlavonoidsAgainst Parkinson's Disease.
Singh NK, Singh A, Mayank. Singh NK, et al. Comb Chem High Throughput Screen. 2024;27(14):2062-2077. doi: 10.2174/0113862073295568240105025006. Comb Chem High Throughput Screen. 2024. PMID: 38243959 Review. - Does lipopolysaccharide-based neuroinflammation induce microglia polarization?
Hernandez Baltazar D, Nadella R, Barrientos Bonilla A, Flores Martínez Y, Olguín A, Heman Bozadas P, Rovirosa Hernández M, Cibrián Llanderal I. Hernandez Baltazar D, et al. Folia Neuropathol. 2020;58(2):113-122. doi: 10.5114/fn.2020.96755. Folia Neuropathol. 2020. PMID: 32729290 Review.
Cited by
- Ginsenoside Rg1 in neurological diseases: From bench to bedside.
Yang SJ, Wang JJ, Cheng P, Chen LX, Hu JM, Zhu GQ. Yang SJ, et al. Acta Pharmacol Sin. 2023 May;44(5):913-930. doi: 10.1038/s41401-022-01022-1. Epub 2022 Nov 15. Acta Pharmacol Sin. 2023. PMID: 36380226 Free PMC article. Review. - miR-505-5p alleviates acute rejection of liver transplantation by inhibiting Myd88 and inducing M2 polarizationof Kupffer cells.
Chai H, Lei Z, Liu Y, Gong J, Cao Z, Huang Z, Yang H, Wu Z. Chai H, et al. Acta Biochim Biophys Sin (Shanghai). 2022 Aug 25;54(8):1148-1158. doi: 10.3724/abbs.2022100. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35959879 Free PMC article. - Do Naturally Occurring Antioxidants Protect Against Neurodegeneration of the Dopaminergic System? A Systematic Revision in Animal Models of Parkinson's Disease.
Costas C, Faro LRF. Costas C, et al. Curr Neuropharmacol. 2022;20(2):432-459. doi: 10.2174/1570159X19666210421092725. Curr Neuropharmacol. 2022. PMID: 33882808 Free PMC article. - Olfactory dysfunction and its related molecular mechanisms in Parkinson's disease.
Gu Y, Zhang J, Zhao X, Nie W, Xu X, Liu M, Zhang X. Gu Y, et al. Neural Regen Res. 2024 Mar;19(3):583-590. doi: 10.4103/1673-5374.380875. Neural Regen Res. 2024. PMID: 37721288 Free PMC article. Review. - Ginsenoside Rg1 alleviates lipopolysaccharide-induced neuronal damage by inhibiting NLRP1 inflammasomes in HT22 cells.
Zhang Y, Ding S, Chen Y, Sun Z, Zhang J, Han Y, Dong X, Fang Z, Li W. Zhang Y, et al. Exp Ther Med. 2021 Jul;22(1):782. doi: 10.3892/etm.2021.10214. Epub 2021 May 19. Exp Ther Med. 2021. PMID: 34055081 Free PMC article.
References
- Iarlori C. Anti-inflammatory agents in Parkinson’s disease. Former Curr Medicinal. 2009;8:72–84.
- Lu L. Novel anti-inflammatory and neuroprotective agents for parkinsons disease. CNS Neurol Disord Drug Targets. 2010;9:232–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous