Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular - PubMed (original) (raw)
Review
Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular
Sandra Buratta et al. Int J Mol Sci. 2020.
Abstract
Beyond the consolidated role in degrading and recycling cellular waste, the autophagic- and endo-lysosomal systems play a crucial role in extracellular release pathways. Lysosomal exocytosis is a process leading to the secretion of lysosomal content upon lysosome fusion with plasma membrane and is an important mechanism of cellular clearance, necessary to maintain cell fitness. Exosomes are a class of extracellular vesicles originating from the inward budding of the membrane of late endosomes, which may not fuse with lysosomes but be released extracellularly upon exocytosis. In addition to garbage disposal tools, they are now considered a cell-to-cell communication mechanism. Autophagy is a cellular process leading to sequestration of cytosolic cargoes for their degradation within lysosomes. However, the autophagic machinery is also involved in unconventional protein secretion and autophagy-dependent secretion, which are fundamental mechanisms for toxic protein disposal, immune signalling and pathogen surveillance. These cellular processes underline the crosstalk between the autophagic and the endosomal system and indicate an intersection between degradative and secretory functions. Further, they suggest that the molecular mechanisms underlying fusion, either with lysosomes or plasma membrane, are key determinants to maintain cell homeostasis upon stressing stimuli. When they fail, the accumulation of undigested substrates leads to pathological consequences, as indicated by the involvement of autophagic and lysosomal alteration in human diseases, namely lysosomal storage disorders, age-related neurodegenerative diseases and cancer. In this paper, we reviewed the current knowledge on the functional role of extracellular release pathways involving lysosomes and the autophagic- and endo-lysosomal systems, evaluating their implication in health and disease.
Keywords: amphisomes; autophagosomes; exosomes; extracellular vesicles; lysosomal exocytosis; secretory autophagy; unconventional protein secretion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
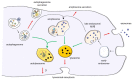
Figure 1
Overview of lysosomal exocytosis, exosome release and autophagy-dependent secretory pathways. Lysosomal exocytosis leads to the secretion of lysosomal content upon lysosome fusion with plasma membrane. Exosomes originate from the inward budding of late endosome membrane, which originates MVBs. They are either released extracellularly upon exocytosis or degraded into lysosomes. Autophagy is a cellular process leading to sequestration of cytosolic cargoes for their degradation within lysosomes. However, the autophagic machinery is also involved in autophagy-dependent secretion of autophagosomes. In addition to merging with lysosomes or plasma membrane, autophagosomes can also fuse with late endosomes/MVBs to produce amphisomes. In turn, amphisomes can either fuse with lysosomes to degrade their content or with plasma membrane. The red arrows indicate fusion with plasma membrane, the green arrows, fusion with lysosome, and the black arrows, pathways leading to organelle maturation and to the intersection between autophagic and endocytic pathway.
Similar articles
- Current knowledge on exosome biogenesis and release.
Hessvik NP, Llorente A. Hessvik NP, et al. Cell Mol Life Sci. 2018 Jan;75(2):193-208. doi: 10.1007/s00018-017-2595-9. Epub 2017 Jul 21. Cell Mol Life Sci. 2018. PMID: 28733901 Free PMC article. Review. - Lysosomal Exocytosis: The Extracellular Role of an Intracellular Organelle.
Tancini B, Buratta S, Delo F, Sagini K, Chiaradia E, Pellegrino RM, Emiliani C, Urbanelli L. Tancini B, et al. Membranes (Basel). 2020 Dec 9;10(12):406. doi: 10.3390/membranes10120406. Membranes (Basel). 2020. PMID: 33316913 Free PMC article. Review. - Insight into the Role of Extracellular Vesicles in Lysosomal Storage Disorders.
Tancini B, Buratta S, Sagini K, Costanzi E, Delo F, Urbanelli L, Emiliani C. Tancini B, et al. Genes (Basel). 2019 Jul 6;10(7):510. doi: 10.3390/genes10070510. Genes (Basel). 2019. PMID: 31284546 Free PMC article. Review. - Tetraspanin CD63 Bridges Autophagic and Endosomal Processes To Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1.
Hurwitz SN, Cheerathodi MR, Nkosi D, York SB, Meckes DG Jr. Hurwitz SN, et al. J Virol. 2018 Feb 12;92(5):e01969-17. doi: 10.1128/JVI.01969-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29212935 Free PMC article. - Synergies in exosomes and autophagy pathways for cellular homeostasis and metastasis of tumor cells.
Salimi L, Akbari A, Jabbari N, Mojarad B, Vahhabi A, Szafert S, Kalashani SA, Soraya H, Nawaz M, Rezaie J. Salimi L, et al. Cell Biosci. 2020 May 13;10:64. doi: 10.1186/s13578-020-00426-y. eCollection 2020. Cell Biosci. 2020. PMID: 32426106 Free PMC article. Review.
Cited by
- Red light mediates the exocytosis of vasodilatory vesicles from cultured endothelial cells: a cellular, and ex vivo murine model.
Weihrauch D, Keszler A, Broeckel G, Aranda E, Lindemer B, Lohr NL. Weihrauch D, et al. Photochem Photobiol Sci. 2024 Feb;23(2):355-364. doi: 10.1007/s43630-023-00522-1. Epub 2024 Jan 26. Photochem Photobiol Sci. 2024. PMID: 38277065 Free PMC article. - The Role of Peroxiredoxins in the Regulation of Sepsis.
Aki T, Unuma K, Uemura K. Aki T, et al. Antioxidants (Basel). 2022 Jan 6;11(1):126. doi: 10.3390/antiox11010126. Antioxidants (Basel). 2022. PMID: 35052630 Free PMC article. Review. - Deciphering _Staphylococcus aureus-_host dynamics using dual activity-based protein profiling of ATP-interacting proteins.
Ahator SD, Hegstad K, Lentz CS, Johannessen M. Ahator SD, et al. mSystems. 2024 May 16;9(5):e0017924. doi: 10.1128/msystems.00179-24. Epub 2024 Apr 24. mSystems. 2024. PMID: 38656122 Free PMC article. - Four distinct cytoplasmic structures generate and release specific vesicles, thus opening the way to intercellular communication.
Racchetti G, Meldolesi J. Racchetti G, et al. Extracell Vesicles Circ Nucl Acids. 2023 Mar 15;4(1):44-58. doi: 10.20517/evcna.2023.03. eCollection 2023. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 39698300 Free PMC article. Review. - Lysosomes and Their Role in Regulating the Metabolism of Hematopoietic Stem Cells.
Arif T. Arif T. Biology (Basel). 2022 Sep 27;11(10):1410. doi: 10.3390/biology11101410. Biology (Basel). 2022. PMID: 36290314 Free PMC article. Review.
References
- Andrews N.W. Regulated secretion of conventional lysosomes. Trends Cell Biol. 2000;10:316–321. - PubMed
- Blott E.J., Griffiths G.M. Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 2002;3:122–131. - PubMed
- Lettau M., Schmidt H., Kabelitz D., Janssen O. Secretory lysosomes and their cargo in T and NK cells. Immunol. Lett. 2007;108:10–19. - PubMed
- Reddy A., Caler E.V., Andrews N.W. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources