Dual Role of Hydrogen Peroxide as an Oxidant in Pneumococcal Pneumonia - PubMed (original) (raw)
Review
. 2021 Apr 20;34(12):962-978.
doi: 10.1089/ars.2019.7964. Epub 2020 Aug 14.
Haroldo A Toque 2 3, Luigi La Pietra 1, Juerg Hamacher 4 5 6, Tenzing Phanthok 7, Alexander Verin 2 7, Joyce Gonzales 7, Yunchao Su 3, David Fulton 2 3, Douglas C Eaton 8, Trinad Chakraborty 1, Rudolf Lucas 2 3 7
Affiliations
- PMID: 32283950
- PMCID: PMC8035917
- DOI: 10.1089/ars.2019.7964
Review
Dual Role of Hydrogen Peroxide as an Oxidant in Pneumococcal Pneumonia
Mobarak Abu Mraheil et al. Antioxid Redox Signal. 2021.
Abstract
Significance:Streptococcus pneumoniae (Spn), a facultative anaerobic Gram-positive human pathogen with increasing rates of penicillin and macrolide resistance, is a major cause of lower respiratory tract infections worldwide. Pneumococci are a primary agent of severe pneumonia in children younger than 5 years and of community-acquired pneumonia in adults. A major defense mechanism toward Spn is the generation of reactive oxygen species, including hydrogen peroxide (H2O2), during the oxidative burst of neutrophils and macrophages. Paradoxically, Spn produces high endogenous levels of H2O2 as a strategy to promote colonization. Recent Advances: Pneumococci, which express neither catalase nor common regulators of peroxide stress resistance, have developed unique mechanisms to protect themselves from H2O2. Spn generates high levels of H2O2 as a strategy to promote colonization. Production of H2O2 moreover constitutes an important virulence phenotype and its cellular activities overlap and complement those of other virulence factors, such as pneumolysin, in modulating host immune responses and promoting organ injury. Critical Issues: This review examines the dual role of H2O2 in pneumococcal pneumonia, from the viewpoint of both the pathogen (defense mechanisms, lytic activity toward competing pathogens, and virulence) and the resulting host-response (inflammasome activation, endoplasmic reticulum stress, and damage to the alveolar-capillary barrier in the lungs). Future Directions: An understanding of the complexity of H2O2-mediated host-pathogen interactions is necessary to develop novel strategies that target these processes to enhance lung function during severe pneumonia.
Keywords: ARDS; hydrogen peroxide; pneumococci; pneumonia; pyruvate oxidase; virulence factor.
Conflict of interest statement
R.L. is inventor on patents related to the use of the TIP peptide in ARDS. The other authors have no conflict of interest.
Figures
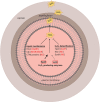
FIG. 1.
Mechanisms of pneumococcal defense against H2O2. Schematic overview of the three defense mechanisms involved in the resistance against H2O2: (i) defense enzymes that directly or indirectly degrade H2O2, (ii) repair mechanisms, and (iii) regulators linked to H2O2 stress response. H2O2, hydrogen peroxide. Color images are available online.
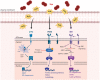
FIG. 2.
Actions of _Spn_-derived H2O2 on the UPR in host cells. Infection with Spn induces the activation of the UPR. Secretion of _Spn_-derived H2O2 leads to activation of PERK, ATF-6, and IRE1. Dimerization and phosphorylation of activated PERK induce phosphorylation of eIF2α leading to inhibition of protein translation and ATF4 modulating expression of target genes. Activated ATF-6 translocates to the Golgi, where it is cleaved by S1P and S2P. The processed ATF-6 enters the nucleus acting as a transcription factor of target genes. Activation of IRE1 leads to splicing of xbp1 mRNA, which acts as transcription factor of target genes. IRE1 activation can also lead to RIDD of mRNA or JNK signaling activation. ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; eIF2α, eukaryotic translation initiation factor 2α; ER, endoplasmic reticulum; IRE1, inositol-requiring enzyme 1; JNK, c-Jun N-terminal kinase; PERK, protein kinase R (PKR)-like ER kinase; RIDD, regulated IRE1-dependend decay; S1P, site-1-protease; S2P, site-1-protease; Spn, Streptococcus pneumonia; UPR, unfolded protein response; xbp1, X-box-binding protein 1. Color images are available online.
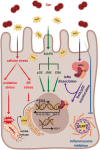
FIG. 3.
H2O2-induced responses in the host cell. Spn produces large amounts of H2O2, which induce a plethora of host cell responses. This includes oxidative and ER stress (red), activation of all three MAPK subfamilies (green), translocation of NF-κB-complex into the nucleus (violet), and inflammasome inhibition (blue). Oxidative stress leads to production of mtROS and release of mtDNA and subsequent STING-dependent type I IFN (IFN-β) expression. Translocation of NF-κB-complex into the nucleus leads to expression of proinflammatory chemokines and cytokines (such as IL-8 and IL23a) and H2O2-dependent production of Nrf-2, which induces expression of HO-1. HO-1, heme oxygenase 1; IFN, interferon; IL, interleukin; MAPK, mitogen-activated protein kinase; mtDNA, mitochondrial DNA; mtROS, mitochondrial reactive oxygen species; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; STING, stimulator of interferon genes. Color images are available online.
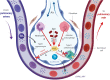
FIG. 4.
The complex actions of H2O2 in alveoli during pneumococcal pneumonia. Spn will migrate into the lower respiratory tract, and since it lacks catalase will generate millimolar levels of H2O2, through the actions of pyruvate oxidase (SpxB) and lactate oxidase (LctO), which will diffuse into the alveolar space (1). Moreover, an early neutrophil-mediated and a later macrophage-derived generation and secretion of μmolar levels of H2O2 will occur (2, 3). High ROS levels in the alveolar space will promote alveolar endothelial barrier function (4) and will impair AFC (5), which is mainly mediated through vectorial sodium transport, involving the apically expressed ENaC and the basolateral Na+-K+ pump in type II pneumocytes. AFC, alveolar fluid clearance; ENaC, epithelial sodium channel; ROS, reactive oxygen species. Color images are available online.
FIG. 5.
Overview of positive (green) and negative (red) actions of endogenous and secreted H2O2 in the pathogen and in the host lung during pneumococcal pneumonia. In Spn, endogenous H2O2 promotes nucleotide, glycolytic, and capsule biosynthesis and increases Ply secretion. Secreted H2O2 kills competing pathogens in the respiratory tract and serves as a virulence factor, since it reduces NLRP3 inflammasome activation in the host. In the alveolar space, _Spn-_secreted H2O2 can induce activation of the UPR and cause DNA damage, which can precede apoptosis. Moreover, H2O2 can impair the alveolar–capillary barrier, induce inflammation, and blunt vectorial Na+ transport, crucial for AFC in pneumocytes. All of these actions of H2O2 on the host lung cells can promote the development of pulmonary edema during pneumococcal pneumonia. Ply, pneumolysin. Color images are available online.
Similar articles
- Streptococcus pneumoniae and Its Virulence Factors H2O2 and Pneumolysin Are Potent Mediators of the Acute Chest Syndrome in Sickle Cell Disease.
Gonzales J, Chakraborty T, Romero M, Mraheil MA, Kutlar A, Pace B, Lucas R. Gonzales J, et al. Toxins (Basel). 2021 Feb 17;13(2):157. doi: 10.3390/toxins13020157. Toxins (Basel). 2021. PMID: 33671422 Free PMC article. Review. - Streptococcus pneumoniae-Induced Oxidative Stress in Lung Epithelial Cells Depends on Pneumococcal Autolysis and Is Reversible by Resveratrol.
Zahlten J, Kim YJ, Doehn JM, Pribyl T, Hocke AC, García P, Hammerschmidt S, Suttorp N, Hippenstiel S, Hübner RH. Zahlten J, et al. J Infect Dis. 2015 Jun 1;211(11):1822-30. doi: 10.1093/infdis/jiu806. Epub 2014 Dec 15. J Infect Dis. 2015. PMID: 25512625 - A Novel Aquaporin Subfamily Imports Oxygen and Contributes to Pneumococcal Virulence by Controlling the Production and Release of Virulence Factors.
Hu Q, Tong H, Wang J, Ge P, Zhu L, Liu C, Zhang JR, Dong X. Hu Q, et al. mBio. 2021 Aug 31;12(4):e0130921. doi: 10.1128/mBio.01309-21. Epub 2021 Aug 17. mBio. 2021. PMID: 34399618 Free PMC article. - Streptococcus pneumoniae Binds to Host Lactate Dehydrogenase via PspA and PspC To Enhance Virulence.
Park SS, Gonzalez-Juarbe N, Martínez E, Hale JY, Lin YH, Huffines JT, Kruckow KL, Briles DE, Orihuela CJ. Park SS, et al. mBio. 2021 May 4;12(3):e00673-21. doi: 10.1128/mBio.00673-21. mBio. 2021. PMID: 33947761 Free PMC article. - Drug treatment of pneumococcal pneumonia in the elderly.
Neralla S, Meyer KC. Neralla S, et al. Drugs Aging. 2004;21(13):851-64. doi: 10.2165/00002512-200421130-00003. Drugs Aging. 2004. PMID: 15493950 Review.
Cited by
- Oxidation of hemoproteins by Streptococcus pneumoniae collapses the cell cytoskeleton and disrupts mitochondrial respiration leading to the cytotoxicity of human lung cells.
Scasny A, Alibayov B, Khan F, Rao SJ, Murin L, Jop Vidal AG, Smith P, Li W, Edwards K, Warncke K, Vidal JE. Scasny A, et al. Microbiol Spectr. 2024 Jan 11;12(1):e0291223. doi: 10.1128/spectrum.02912-23. Epub 2023 Dec 12. Microbiol Spectr. 2024. PMID: 38084982 Free PMC article. - A maternal high-fat/low-fiber diet impairs glucose tolerance and induces the formation of glycolytic muscle fibers in neonatal offspring.
Hu C, Yang Y, Chen M, Hao X, Wang S, Yang L, Yin Y, Tan C. Hu C, et al. Eur J Nutr. 2021 Aug;60(5):2709-2718. doi: 10.1007/s00394-020-02461-4. Epub 2021 Jan 2. Eur J Nutr. 2021. PMID: 33386892 - Streptococcus pneumoniae and Its Virulence Factors H2O2 and Pneumolysin Are Potent Mediators of the Acute Chest Syndrome in Sickle Cell Disease.
Gonzales J, Chakraborty T, Romero M, Mraheil MA, Kutlar A, Pace B, Lucas R. Gonzales J, et al. Toxins (Basel). 2021 Feb 17;13(2):157. doi: 10.3390/toxins13020157. Toxins (Basel). 2021. PMID: 33671422 Free PMC article. Review. - Antioxidants as Therapeutic Agents in Acute Respiratory Distress Syndrome (ARDS) Treatment-From Mice to Men.
von Knethen A, Heinicke U, Laux V, Parnham MJ, Steinbicker AU, Zacharowski K. von Knethen A, et al. Biomedicines. 2022 Jan 4;10(1):98. doi: 10.3390/biomedicines10010098. Biomedicines. 2022. PMID: 35052778 Free PMC article. Review. - Impact of Endogenous Pneumococcal Hydrogen Peroxide on the Activity and Release of Pneumolysin.
Bazant J, Ott B, Hudel M, Hain T, Lucas R, Mraheil MA. Bazant J, et al. Toxins (Basel). 2023 Sep 30;15(10):593. doi: 10.3390/toxins15100593. Toxins (Basel). 2023. PMID: 37888624 Free PMC article.
References
- Anderson R and Feldman C. Review manuscript: mechanisms of platelet activation by the pneumococcus and the role of platelets in community-acquired pneumonia. J Infect 75: 473–485, 2017 - PubMed
- Anderson R, Steel HC, Cockeran R, von Gottberg A, de Gouveia L, Klugman KP, Mitchell TJ, and Feldman C. Comparison of the effects of macrolides, amoxicillin, ceftriaxone, doxycycline, tobramycin and fluoroquinolones, on the production of pneumolysin by Streptococcus pneumoniae in vitro. J Antimicrob Chemother 60: 1155–1158, 2007 - PubMed
- Auzat I, Chapuy-Regaud S, Le Bras G, Dos Santos D, Ogunniyi AD, Le Thomas I, Garel JR, Paton JC, and Trombe MC. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol Microbiol 34: 1018–1028, 1999 - PubMed
- Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, and Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical