Modulators of microglia activation and polarization in ischemic stroke (Review) - PubMed (original) (raw)
Review
Modulators of microglia activation and polarization in ischemic stroke (Review)
Cheng-Ting Jiang et al. Mol Med Rep. 2020 May.
Abstract
Ischemic stroke is one of the leading causes of mortality and disability worldwide. However, there is a current lack of effective therapies available. As the resident macrophages of the brain, microglia can monitor the microenvironment and initiate immune responses. In response to various brain injuries, such as ischemic stroke, microglia are activated and polarized into the proinflammatory M1 phenotype or the anti‑inflammatory M2 phenotype. The immunomodulatory molecules, such as cytokines and chemokines, generated by these microglia are closely associated with secondary brain damage or repair, respectively, following ischemic stroke. It has been shown that M1 microglia promote secondary brain damage, whilst M2 microglia facilitate recovery following stroke. In addition, autophagy is also reportedly involved in the pathology of ischemic stroke through regulating the activation and function of microglia. Therefore, this review aimed to provide a comprehensive overview of microglia activation, their functions and changes, and the modulators of these processes, including transcription factors, membrane receptors, ion channel proteins and genes, in ischemic stroke. The effects of autophagy on microglia polarization in ischemic stroke were also reviewed. Finally, future research areas of ischemic stroke and the implications of the current knowledge for the development of novel therapeutics for ischemic stroke were identified.
Keywords: microglia; ischemic stroke; polarization; autophagy; immune responses; pathology; modulation mechanisms.
Figures
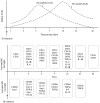
Figure 1.
Dynamic changes in microglia marker levels over time following IS. Top panel: M1 microglia markers demonstrated an increasing trend during the first 14 days following IS, after which they decreased. Expression levels of M2 microglia markers increased from day 1, peaked at days 5–7 and decreased until day 42. Bottom panel: Microglia exhibited an M2-like response as early as 1 day following IS, which manifested as increased expression levels of CD206 Arg-1. The expression levels of TGF-β increased from day 3 until day 21. With regards to the M1 markers, the expression levels of the proinflammatory cytokines, TNF-α, IL-6 and IL-1β increased from day 3. The levels of M1 surface markers CD16 and CD32 increased from day 1 and CD16 and iNOS expression levels remained increased until day 42 after IS. IS, ischemic stroke; Arg-1, arginase 1; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor α; IL, interleukin; iNOS, inducible nitric oxide synthase; CCL, C-C motif chemokine ligand; Ym1/2, chitinase-3-like protein 3.
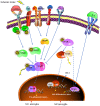
Figure 2.
Modulatory mechanisms of microglia polarization following ischemic stroke. Microglia are activated following ischemic stroke. M1 microglia activation involves different factors, including the cytokines S1P, LPS, HMGB1, S100B and CKLF1. These factors bind to membrane receptors, such as the cytokine receptors S1PR, RAGE, TLR4, IL-4R and CCR4, to trigger proinflammatory cellular signaling pathways. These pathways include the JAK2/STAT1/NF-κB, JAK2/STAT3/NF-κB and MyD88/NF-κB pathways. The downstream target NF-κB is released and translocates from the cytoplasm to the nucleus following the phosphorylation of IκB, where it initiates the transcription of proinflammatory genes. M2 microglia can be activated by IL-4, which promotes PPARγ mobilization from the nucleus to the cytoplasm. Subsequently, PPARγ inhibits the activation of NF-κB and promotes the activation of Nrf2 to induce the transcription of anti-inflammatory genes. Additionally, the activated Nrf2 promotes the expression of HO-1, which scavenges reactive oxygen species and nitric oxide. S1P, sphingosine 1 phosphate; LPS, lipopolysaccharide; IL, interleukin; HMGB1, high mobility group protein B1; S100B, protein S100-B; CKLF1, chemokine-like factor; SIPR, sphingosine 1 phosphate receptor; TRL4, toll-like receptor 4; CCR4, C-C chemokine receptor type 4; RAGE; JAK2, Janus activated kinase 2; MyD88, myeloid differentiation primary response protein MyD88; IκB, NF-κB inhibitor; PPARγ, peroxisome proliferator-activated receptor γ; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase 1; Keap 1, Kelch-like ECH-associated protein 1; STAT, signal transducer and activator of transcription.
Similar articles
- Research progress on mechanisms of ischemic stroke: Regulatory pathways involving Microglia.
Gao X, Su G, Chai M, Shen M, Hu Z, Chen W, Gao J, Li R, Ma T, An Y, Zhang Z. Gao X, et al. Neurochem Int. 2024 Jan;172:105656. doi: 10.1016/j.neuint.2023.105656. Epub 2023 Dec 9. Neurochem Int. 2024. PMID: 38081419 - Minocycline promotes functional recovery in ischemic stroke by modulating microglia polarization through STAT1/STAT6 pathways.
Lu Y, Zhou M, Li Y, Li Y, Hua Y, Fan Y. Lu Y, et al. Biochem Pharmacol. 2021 Apr;186:114464. doi: 10.1016/j.bcp.2021.114464. Epub 2021 Feb 10. Biochem Pharmacol. 2021. PMID: 33577892 - The role of microglial activation on ischemic stroke: Modulation by fibroblast growth factors.
Dordoe C, Huang W, Bwalya C, Wang X, Shen B, Wang H, Wang J, Ye S, Wang P, Xiaoyan B, Li X, Lin L. Dordoe C, et al. Cytokine Growth Factor Rev. 2023 Dec;74:122-133. doi: 10.1016/j.cytogfr.2023.07.005. Epub 2023 Jul 31. Cytokine Growth Factor Rev. 2023. PMID: 37573252 Review. - Treatment targets for M2 microglia polarization in ischemic stroke.
Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y. Wang J, et al. Biomed Pharmacother. 2018 Sep;105:518-525. doi: 10.1016/j.biopha.2018.05.143. Epub 2018 Jun 6. Biomed Pharmacother. 2018. PMID: 29883947 Review.
Cited by
- Systemic immune responses after ischemic stroke: From the center to the periphery.
Wu F, Liu Z, Zhou L, Ye D, Zhu Y, Huang K, Weng Y, Xiong X, Zhan R, Shen J. Wu F, et al. Front Immunol. 2022 Sep 20;13:911661. doi: 10.3389/fimmu.2022.911661. eCollection 2022. Front Immunol. 2022. PMID: 36211352 Free PMC article. Review. - Exosomal miRNAs as Biomarkers of Ischemic Stroke.
Ciaccio AM, Tuttolomondo A. Ciaccio AM, et al. Brain Sci. 2023 Nov 27;13(12):1647. doi: 10.3390/brainsci13121647. Brain Sci. 2023. PMID: 38137095 Free PMC article. Review. - Protective role of activating transcription factor 3 against neuronal damage in rats with cerebral ischemia.
Ma N, Li G, Fu X. Ma N, et al. Brain Behav. 2022 Apr;12(4):e2522. doi: 10.1002/brb3.2522. Epub 2022 Mar 8. Brain Behav. 2022. PMID: 35263513 Free PMC article. - Adenosine A2A Receptor in Bone Marrow-Derived Cells Mediated Macrophages M2 Polarization via PPARγ-P65 Pathway in Chronic Hypoperfusion Situation.
Mou KJ, Shen KF, Li YL, Wu ZF, Duan W. Mou KJ, et al. Front Aging Neurosci. 2022 Jan 3;13:792733. doi: 10.3389/fnagi.2021.792733. eCollection 2021. Front Aging Neurosci. 2022. PMID: 35046793 Free PMC article. - MLIF Modulates Microglia Polarization in Ischemic Stroke by Targeting eEF1A1.
Liu Y, Deng S, Song Z, Zhang Q, Guo Y, Yu Y, Wang Y, Li T, Megahed FAK, Addissouky TA, Mao J, Zhang Y. Liu Y, et al. Front Pharmacol. 2021 Sep 7;12:725268. doi: 10.3389/fphar.2021.725268. eCollection 2021. Front Pharmacol. 2021. PMID: 34557098 Free PMC article.
References
- World Health Organization (WHO), corp-author WHO; Geneva: 2016. [May 24;2018 ]. Top 10 global causes of deaths, 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials