Stealth Coating of Nanoparticles in Drug-Delivery Systems - PubMed (original) (raw)
Review
Stealth Coating of Nanoparticles in Drug-Delivery Systems
See Yee Fam et al. Nanomaterials (Basel). 2020.
Abstract
Nanoparticles (NPs) have emerged as a powerful drug-delivery tool for cancer therapies to enhance the specificity of drug actions, while reducing the systemic side effects. Nonetheless, NPs interact massively with the surrounding physiological environments including plasma proteins upon administration into the bloodstream. Consequently, they are rapidly cleared from the blood circulation by the mononuclear phagocyte system (MPS) or complement system, resulting in a premature elimination that will cause the drug release at off-target sites. By grafting a stealth coating layer onto the surface of NPs, the blood circulation half-life of nanomaterials can be improved by escaping the recognition and clearance of the immune system. This review focuses on the basic concept underlying the stealth behavior of NPs by polymer coating, whereby the fundamental surface coating characteristics such as molecular weight, surface chain density as well as conformations of polymer chains are of utmost importance for efficient protection of NPs. In addition, the most commonly used stealth polymers such as poly(ethylene glycol) (PEG), poly(2-oxazoline) (POx), and poly(zwitterions) in developing long-circulating NPs for drug delivery are also thoroughly discussed. The biomimetic strategies, including the cell-membrane camouflaging technique and CD47 functionalization for the development of stealth nano-delivery systems, are highlighted in this review as well.
Keywords: drug delivery; nanoparticles; opsonization; phagocytosis; polymer; stealth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
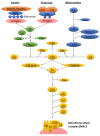
A simplified overview of different activation pathways of the complement system. There are three complement activation pathways: the classical pathway, which is activated by antibody binding or the direct fixation of complement component C1q bound to zymogens C1r and C1s on the surface of an antigen; the lectin pathway, which is triggered by the binding of mannan-binding lectin (MBL) activated by MBL-associated serine proteases (MASP), namely MASP1 and MASP2, to mannose contained on the surface of an antigen; and the alternative pathway, which is triggered directly by the binding of spontaneously activated complement component on the surface of an antigen. The complement enzymatic cascades of each pathway generate a key protease called C3 convertase that cleaves C3 into C3b and C3a. This complement activation leads to eventual antigen opsonization, inflammatory responses, and membrane lysis.
Figure 2
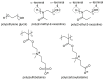
Chemical structures of the stealth polymers. The alpha (α) and omega (ω) termini of poly(2-methyl-2-oxazoline) and poly(2-ethyl-2-oxazoline) are indicated in circles.
Figure 3
Schematic representations of CD47 regulation on phagocytosis of nanoparticles (NPs). (a) CD47 coated on a nanoparticle interacts with the signal regulatory protein alpha (SIRPα) expressed on the surface of the macrophage, triggering a potent “don’t-eat-me” signal, which inhibits phagocytosis; (b) A nanoparticle without CD47 functionalization is recognized by macrophage for particle engulfment and phagocytosis.
Figure 4
Schematic representations of poly(ethylene glycol) (PEG) conformations on NPs. (a) At low surface coverage, PEG chains are located closer to the particle’s surface, leading to a mushroom conformation; (b) At high surface coverage, PEG chains are lack of mobility and extended away from the particle’s surface, leading to a brush conformation. RF represents the Flory radius of the PEG graft; D represents the distance between the adjacent PEG grafts; L represents the thickness of the grafted PEG layer (the diagrams are drawn not to scale).
Similar articles
- The Fate of Nanoparticles In Vivo and the Strategy of Designing Stealth Nanoparticle for Drug Delivery.
Bao J, Zhang Q, Duan T, Hu R, Tang J. Bao J, et al. Curr Drug Targets. 2021;22(8):922-946. doi: 10.2174/1389450122666210118105122. Curr Drug Targets. 2021. PMID: 33461465 Review. - CD47 Functionalization of Nanoparticles as a Poly(ethylene glycol) Alternative: A Novel Approach to Improve Drug Delivery.
Vandchali NR, Moadab F, Taghizadeh E, Tajbakhsh A, Gheibihayat SM. Vandchali NR, et al. Curr Drug Targets. 2021;22(15):1750-1759. doi: 10.2174/1389450122666210204203514. Curr Drug Targets. 2021. PMID: 33563192 Review. - Stealth properties to improve therapeutic efficacy of drug nanocarriers.
Salmaso S, Caliceti P. Salmaso S, et al. J Drug Deliv. 2013;2013:374252. doi: 10.1155/2013/374252. Epub 2013 Mar 7. J Drug Deliv. 2013. PMID: 23533769 Free PMC article. - Bioinspired "Active" Stealth Magneto-Nanomicelles for Theranostics Combining Efficient MRI and Enhanced Drug Delivery.
Zhang KL, Zhou J, Zhou H, Wu Y, Liu R, Wang LL, Lin WW, Huang G, Yang HH. Zhang KL, et al. ACS Appl Mater Interfaces. 2017 Sep 13;9(36):30502-30509. doi: 10.1021/acsami.7b10086. Epub 2017 Aug 30. ACS Appl Mater Interfaces. 2017. PMID: 28812358 - C1q-Mediated Complement Activation and C3 Opsonization Trigger Recognition of Stealth Poly(2-methyl-2-oxazoline)-Coated Silica Nanoparticles by Human Phagocytes.
Tavano R, Gabrielli L, Lubian E, Fedeli C, Visentin S, Polverino De Laureto P, Arrigoni G, Geffner-Smith A, Chen F, Simberg D, Morgese G, Benetti EM, Wu L, Moghimi SM, Mancin F, Papini E. Tavano R, et al. ACS Nano. 2018 Jun 26;12(6):5834-5847. doi: 10.1021/acsnano.8b01806. Epub 2018 May 23. ACS Nano. 2018. PMID: 29750504 Free PMC article.
Cited by
- Capped Plasmonic Gold and Silver Nanoparticles with Porphyrins for Potential Use as Anticancer Agents-A Review.
Hlapisi N, Songca SP, Ajibade PA. Hlapisi N, et al. Pharmaceutics. 2024 Sep 28;16(10):1268. doi: 10.3390/pharmaceutics16101268. Pharmaceutics. 2024. PMID: 39458600 Free PMC article. Review. - Hydrolytically Degradable Zwitterionic Polyphosphazene Containing HEPES Moieties as Side Groups.
Tagad HD, Marin A, Hlushko R, Andrianov AK. Tagad HD, et al. Biomacromolecules. 2024 Oct 14;25(10):6791-6800. doi: 10.1021/acs.biomac.4c01008. Epub 2024 Sep 24. Biomacromolecules. 2024. PMID: 39315416 Free PMC article. - PEGylated pH-Responsive Liposomes for Enhancing the Intracellular Uptake and Cytotoxicity of Paclitaxel in MCF-7 Breast Cancer Cells.
Nijhawan HP, Shyamsundar P, Prabhakar B, Yadav KS. Nijhawan HP, et al. AAPS PharmSciTech. 2024 Sep 17;25(7):216. doi: 10.1208/s12249-024-02930-7. AAPS PharmSciTech. 2024. PMID: 39289249 - Macrophage membrane coated discoidal polymeric particles for evading phagocytosis.
Aryal S, Park S, Cho H, Choi KC, Choi MJ, Park YS, Key J. Aryal S, et al. Biomed Eng Lett. 2024 Jun 12;14(5):1113-1124. doi: 10.1007/s13534-024-00396-x. eCollection 2024 Sep. Biomed Eng Lett. 2024. PMID: 39220034 - Insights into the prospects of nanobiomaterials in the treatment of cardiac arrhythmia.
Lu D, Fan X. Lu D, et al. J Nanobiotechnology. 2024 Aug 30;22(1):523. doi: 10.1186/s12951-024-02805-w. J Nanobiotechnology. 2024. PMID: 39215361 Free PMC article. Review.
References
- Chen Q., Ding H., Zhou J., Zhao X., Zhang J., Yang C., Li K., Qiao M., Hu H., Ding P., et al. Novel glycyrrhetinic acid conjugated pH-sensitive liposomes for the delivery of doxorubicin and its antitumor activities. RSC Adv. 2016;6:17782–17791. doi: 10.1039/C6RA01580H. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials