Stress-Induced Morphological, Cellular and Molecular Changes in the Brain-Lessons Learned from the Chronic Mild Stress Model of Depression - PubMed (original) (raw)
Review
Stress-Induced Morphological, Cellular and Molecular Changes in the Brain-Lessons Learned from the Chronic Mild Stress Model of Depression
Ahmad Raza Khan et al. Cells. 2020.
Abstract
Major depressive disorder (MDD) is a severe illness imposing an increasing social and economic burden worldwide. Numerous rodent models have been developed to investigate the pathophysiology of MDD. One of the best characterized and most widely used models is the chronic mild stress (CMS) model which was developed more than 30 years ago by Paul Willner. More than 2000 published studies used this model, mainly to assess novel compounds with potential antidepressant efficacy. Most of these studies examined the behavioral consequences of stress and concomitant drug intervention. Much fewer studies focused on the CMS-induced neurobiological changes. However, the stress-induced cellular and molecular changes are important as they may serve as potential translational biomarkers and increase our understanding of the pathophysiology of MDD. Here, we summarize current knowledge on the structural and molecular alterations in the brain that have been described using the CMS model. We discuss the latest neuroimaging and postmortem histopathological data as well as molecular changes including recent findings on microRNA levels. Different chronic stress paradigms occasionally deliver dissimilar findings, but the available experimental data provide convincing evidence that the CMS model has a high translational value. Future studies examining the neurobiological changes in the CMS model in combination with clinically effective antidepressant drug intervention will likely deliver further valuable information on the pathophysiology of MDD.
Keywords: 1H MRS; CMS; MRI; animal model; depressive disorder; diffusion MRI; magnetic resonance imaging; microRNA; neuroplasticity; proton magnetic resonance spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Figures
Figure 1
The weekly protocol of the chronic mild stress (CMS) treatment. Adult male Wistar rats are subjected every day to a different micro-stressor lasting for 10–14 h. Intermittent illumination: lights on and off every 2 h; Cage tilting into a 45° position; Strobe flashing: stroboscopic lightning; Damp bedding: pouring water into the cage to damp the beddings; Paired housing: pairing two rats by having an unfamiliar partner at each grouping session. This weekly schedule is typically repeated over a period of 4–8 weeks.
Figure 2
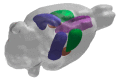
A representative 3D image of a rat brain which has been digitally reconstructed from magnetic resonance imaging (MRI) scans and depicts the medial prefrontal cortex (violet), caudate putamen (green), hippocampus (blue) and amygdala (brown).
Figure 3
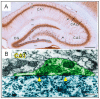
A representative microscopic image of the rat hippocampus (A). In this coronal section, the GABAergic perisomatic inhibitory neurons were visualized with parvalbumin-immunohistochemistry, see the numerous brown cells, some of them are indicated with arrows. Their dense axon fibers delineate the two distinct cell layers (gcl and pyr) formed by the granule cells (gcl) of the dentate gyrus and pyramidal neurons (pyr) of the CA1–3 areas. Abbreviations: o: stratum oriens; pyr: stratum pyramidale; r: stratum radiatum; lm: stratum lacunosum-moleculare; m: dentate molecular layer (stratum moleculare); gcl: granule cell layer (stratum granulosum); h: hilus proper; DG: dentate gyrus; CA1–3: Cornu Ammonis 1–3. A representative electron microscopy image of perisomatic contacts and inhibitory synapses in the CA1 area (B). The axon terminal (green) of an inhibitory neuron projects to the soma (blue) of a pyramidal cell. Synaptic densities are indicated with yellow arrowheads. Scale bars: 0.5 mm on A and 250 nm on B.
Figure 4
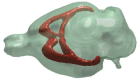
A representative 3D image showing the main commissural white matter pathways (brown) of the rat brain.
Similar articles
- Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm.
Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, Gavioli EC, Dal-Pizzol F, Quevedo J. Lucca G, et al. J Psychiatr Res. 2009 Jun;43(9):864-9. doi: 10.1016/j.jpsychires.2008.11.002. Epub 2008 Dec 19. J Psychiatr Res. 2009. PMID: 19100996 - iTRAQ-based quantitative analysis of hippocampal postsynaptic density-associated proteins in a rat chronic mild stress model of depression.
Han X, Shao W, Liu Z, Fan S, Yu J, Chen J, Qiao R, Zhou J, Xie P. Han X, et al. Neuroscience. 2015 Jul 9;298:220-92. doi: 10.1016/j.neuroscience.2015.04.006. Epub 2015 Apr 9. Neuroscience. 2015. PMID: 25862978 - Neurobiology of chronic mild stress: parallels to major depression.
Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Hill MN, et al. Neurosci Biobehav Rev. 2012 Oct;36(9):2085-117. doi: 10.1016/j.neubiorev.2012.07.001. Epub 2012 Jul 7. Neurosci Biobehav Rev. 2012. PMID: 22776763 Free PMC article. Review. - Treatment with TREK1 and TRPC3/6 ion channel inhibitors upregulates microRNA expression in a mouse model of chronic mild stress.
Buran İ, Etem EÖ, Tektemur A, Elyas H. Buran İ, et al. Neurosci Lett. 2017 Aug 24;656:51-57. doi: 10.1016/j.neulet.2017.07.017. Epub 2017 Jul 14. Neurosci Lett. 2017. PMID: 28716528 - Animal models of major depression and their clinical implications.
Czéh B, Fuchs E, Wiborg O, Simon M. Czéh B, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2016 Jan 4;64:293-310. doi: 10.1016/j.pnpbp.2015.04.004. Epub 2015 Apr 17. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 25891248 Review.
Cited by
- Chronic Stress Alters Astrocyte Morphology in Mouse Prefrontal Cortex.
Codeluppi SA, Chatterjee D, Prevot TD, Bansal Y, Misquitta KA, Sibille E, Banasr M. Codeluppi SA, et al. Int J Neuropsychopharmacol. 2021 Oct 23;24(10):842-853. doi: 10.1093/ijnp/pyab052. Int J Neuropsychopharmacol. 2021. PMID: 34346493 Free PMC article. - Deciphering the links between psychological stress, depression, and neurocognitive decline in patients with Down syndrome.
Poumeaud F, Mircher C, Smith PJ, Faye PA, Sturtz FG. Poumeaud F, et al. Neurobiol Stress. 2021 Feb 5;14:100305. doi: 10.1016/j.ynstr.2021.100305. eCollection 2021 May. Neurobiol Stress. 2021. PMID: 33614867 Free PMC article. Review. - A Preliminary Quantitative Electron Microscopic Analysis Reveals Reduced Number of Mitochondria in the Infralimbic Cortex of Rats Exposed to Chronic Mild Stress.
Csabai D, Sebők-Tornai A, Wiborg O, Czéh B. Csabai D, et al. Front Behav Neurosci. 2022 May 4;16:885849. doi: 10.3389/fnbeh.2022.885849. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35600987 Free PMC article. - What Do the Animal Studies of Stress Resilience Teach Us?
Dziedzicka-Wasylewska M, Solich J, Korlatowicz A, Faron-Górecka A. Dziedzicka-Wasylewska M, et al. Cells. 2021 Jun 29;10(7):1630. doi: 10.3390/cells10071630. Cells. 2021. PMID: 34209787 Free PMC article. Review. - Activation of liver X receptors protects oligodendrocytes in CA3 of stress-induced mice.
Zhu P, Tang J, Liang X, Luo Y, Wang J, Li Y, Xiao K, Li J, Deng Y, Jiang L, Xiao Q, Qi Y, Xie Y, Yang H, Zhu L, Tang Y, Huang C. Zhu P, et al. Front Pharmacol. 2022 Jul 25;13:936045. doi: 10.3389/fphar.2022.936045. eCollection 2022. Front Pharmacol. 2022. PMID: 35959443 Free PMC article.
References
- World Health Assembly . 65 Global Burden of Mental Disorders and the Need for a Comprehensive, Coordinated Response from Health and Social Sectors at the Country Level: Report by the Secretariat. World Health Organization; Geneva, Swizerland: 2012. p. 4.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Volume 991. American Psychiatric Association; Arlington, VA, USA: 2013. DSM-5.
- First M.B. DSM-5® Handbook of Differential Diagnosis. American Psychiatric Publishing; Wasington, DC, USA: 2013.
- Videbech P., Ravnkilde B. Reviews and Overviews Hippocampal Volume and Depression: A Meta-Analysis of MRI Studies. Hippocampal Vol. Depress. A Meta Anal. MRI Stud. 2004;161:1957–1966. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical