Pathophysiological implications of hypoxia in human diseases - PubMed (original) (raw)
Review
Pathophysiological implications of hypoxia in human diseases
Pai-Sheng Chen et al. J Biomed Sci. 2020.
Abstract
Oxygen is essentially required by most eukaryotic organisms as a scavenger to remove harmful electron and hydrogen ions or as a critical substrate to ensure the proper execution of enzymatic reactions. All nucleated cells can sense oxygen concentration and respond to reduced oxygen availability (hypoxia). When oxygen delivery is disrupted or reduced, the organisms will develop numerous adaptive mechanisms to facilitate cells survived in the hypoxic condition. Normally, such hypoxic response will cease when oxygen level is restored. However, the situation becomes complicated if hypoxic stress persists (chronic hypoxia) or cyclic normoxia-hypoxia phenomenon occurs (intermittent hypoxia). A series of chain reaction-like gene expression cascade, termed hypoxia-mediated gene regulatory network, will be initiated under such prolonged or intermittent hypoxic conditions and subsequently leads to alteration of cellular function and/or behaviors. As a result, irreversible processes occur that may cause physiological disorder or even pathological consequences. A growing body of evidence implicates that hypoxia plays critical roles in the pathogenesis of major causes of mortality including cancer, myocardial ischemia, metabolic diseases, and chronic heart and kidney diseases, and in reproductive diseases such as preeclampsia and endometriosis. This review article will summarize current understandings regarding the molecular mechanism of hypoxia in these common and important diseases.
Keywords: Cancer; Cardiomyopathy; Chronic kidney disease; Endometriosis; Hypoxia; Metabolic diseases; Preeclampsia.
Conflict of interest statement
All authors declare there is no conflict of interest.
Figures
Fig. 1
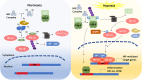
Schematic diagram illustrates the regulation of HIF-1α and NF-κB under normoxic and hypoxic conditions
Fig. 2
Hypoxia-regulated cancer progression. Hypoxia is a typical feature of tumor microenvironment, which contributes to initial tumorigenesis, induced angiogenesis, drug resistance, and cancer metastasis. The major upstream regulators (gray), functional downstream genes (blue), and resulting cellular consequences (yellow) under the control of hypoxia signaling are indicated
Fig. 3
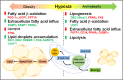
Hypoxia-regulated lipid metabolism related to obesity. Hypoxia is shown as a promoter or a suppressor of obesity by regulating lipid metabolism. The major changes (increases shown in red; decreases shown in green) involved in fatty acid β-oxidation, extracellular fatty acid influx, lipolysis, lipogenesis and lipid droplet accumulation under hypoxia for its promoting obesity or anti-obesity effects are summarized
Fig. 4
Schematic diagram of the hypoxic process linked to kidney diseases. During the development of CKD, vascular endothelial cells died and causing atrophy of microvessels. This will cause local hypoxia between tissues and cause inflammatory reactions. Hypoxia induces the expression of TGF-β, which leads to fibroblast transformation into myofibroblast, increases ECM production, and causes fibrosis. Renal tubular epithelial cells may encounter cell cycle arrest, apoptosis, autophagy, and finally shrink during renal fibrosis
Fig. 5
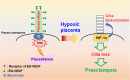
Potential role of hypoxia in developing preeclampsia. Binding of EG-VEGF to its receptor on the primary cilium activates ERK signaling at the basal body for proper placentation. Under hypoxic condition, however, HIF-1α translocates to the base of cilia and induces cilia deacetylation, thus leading to ciliary resorption. The hypoxia-induced ciliary defects contribute to the development of preeclampsia
Fig. 6
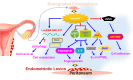
Impacts of hypoxia on endometriosis pathogenesis. Shed-off endometrial tissues will immediately suffer hypoxic stress when it retrogrades into the peritoneal cavity during menstruation. Hypoxia regulates numerous downstream target genes involved in different cellular processes including cell survival, metabolism, angiogenesis, E2 and PGE2 production, and cell adhesion to help the development of endometriosis
Similar articles
- Hypoxia: The force of endometriosis.
Wu MH, Hsiao KY, Tsai SJ. Wu MH, et al. J Obstet Gynaecol Res. 2019 Mar;45(3):532-541. doi: 10.1111/jog.13900. Epub 2019 Jan 7. J Obstet Gynaecol Res. 2019. PMID: 30618168 Review. - Chronic mountain sickness: the reaction of physical disorders to chronic hypoxia.
Zubieta-Castillo G Sr, Zubieta-Calleja GR Jr, Zubieta-Calleja L. Zubieta-Castillo G Sr, et al. J Physiol Pharmacol. 2006 Sep;57 Suppl 4:431-42. J Physiol Pharmacol. 2006. PMID: 17072074 - Recent progress of biocompatible carbon dots in hypoxia-related fields.
Huang Q, Qiao Lv, Jiang L, Chen Q, Zhang K. Huang Q, et al. J Biomater Appl. 2023 Feb;37(7):1159-1168. doi: 10.1177/08853282221125313. Epub 2022 Sep 9. J Biomater Appl. 2023. PMID: 36083209 - Role of exosomes and microvesicles in hypoxia-associated tumour development and cardiovascular disease.
Belting M, Christianson HC. Belting M, et al. J Intern Med. 2015 Sep;278(3):251-63. doi: 10.1111/joim.12393. Epub 2015 Jul 8. J Intern Med. 2015. PMID: 26153525 Review. - [Significance of the hypoxic syndrome in the pathogenesis of diseases of the pregnant mother and child].
Luk'ianova EM. Luk'ianova EM. Fiziol Zh (1978). 1982 Sep-Oct;28(5):522-9. Fiziol Zh (1978). 1982. PMID: 7141021 Russian. No abstract available.
Cited by
- Chemical Hypoxia Induces Pyroptosis in Neuronal Cells by Caspase-Dependent Gasdermin Activation.
Park CH, Park JY, Cho WG. Park CH, et al. Int J Mol Sci. 2024 Feb 11;25(4):2185. doi: 10.3390/ijms25042185. Int J Mol Sci. 2024. PMID: 38396860 Free PMC article. - Hypoxia modeling techniques: A review.
Salyha N, Oliynyk I. Salyha N, et al. Heliyon. 2023 Feb;9(2):e13238. doi: 10.1016/j.heliyon.2023.e13238. Epub 2023 Jan 26. Heliyon. 2023. PMID: 36718422 Free PMC article. Review. - Retinopathy of prematurity: contribution of inflammatory and genetic factors.
Fevereiro-Martins M, Guimarães H, Marques-Neves C, Bicho M. Fevereiro-Martins M, et al. Mol Cell Biochem. 2022 Jun;477(6):1739-1763. doi: 10.1007/s11010-022-04394-4. Epub 2022 Mar 9. Mol Cell Biochem. 2022. PMID: 35262882 Review. - Prognostic value and anti-tumor immunity role of TMED9 in pan-cancer: a bioinformatics study.
Wang H, Wang Y, Tan P, Liu Y, Zhou S, Ma W. Wang H, et al. Transl Cancer Res. 2024 Oct 31;13(10):5429-5445. doi: 10.21037/tcr-24-258. Epub 2024 Sep 11. Transl Cancer Res. 2024. PMID: 39525003 Free PMC article. - Hyperbaric Oxygen Therapy for Complications in Nipple-Sparing Mastectomy with Breast Reconstruction: A Systematic Review.
Idris OA, Ahmedfiqi YO, Shebrain A, Al-Assil T, Pacione SC, Haj D, Motan AD, Momani F, Bzizi H, Jahromi BS, Lewis RM, Steeg KV 2nd. Idris OA, et al. J Clin Med. 2024 Jun 17;13(12):3535. doi: 10.3390/jcm13123535. J Clin Med. 2024. PMID: 38930063 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- NHRI-EX106-10516BI/National Health Research Institutes (TW)
- MOST 106-2320-B-006 -072 -MY3/Ministry of Science and Technology, Taiwan
- MOST 107-2321-B-006-014/Ministry of Science and Technology, Taiwan
LinkOut - more resources
Full Text Sources
Medical
Research Materials